Founder of TriboNet, Editor, PhD (Tribology), Tribology Scientist at ASML, The Netherlands. Expertise in lubrication, friction, wear and contact mechanics with emphasis on modeling. Creator of Tribology Simulator.
The Mechanism of Glycerol Superlubricity
Glycerol is a highly viscous liquid, generating friction coefficient of 0.1 and up for bearing steels in boundary lubrication. In full film EHL, pure glycerol generates high friction as well and therefore is rarely used as a lubricant. It is, on the other hand, non-toxic and bio degradable, hence attractive for use in sensitive applications. Surprisingly, super low friction coefficient (less than 0.01) can be achieved in macro scale by the use of glycerol/water mixture as a lubricant in relatively mild, mixed lubricated steel-steel contacts, as it was discussed earlier. The mechanism leading to the low friction is of great interest.
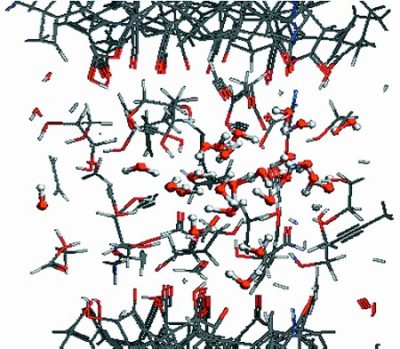
Superlubricity in the contact of DLC coated steel surfaces in boundary lubrication was observed in the presence of glycerol by Matta and colleagues. A hydrogen free ![]() type of DLC coating was considered and the mechanism of the low friction was proposed. By performing experiments and Molecular Dynamic (MD) simulations, the researchers came up with a model of superlubricity by glycerol, consisting of three points. First, a hydroxylation of the contacting solids is forced by a tribochemical reaction, enhanced by the contact pressure and temperature. Second, the glycerol molecules absorb on the OH-terminated surface (due to hydroxylation) by hydrogen bond and create a tribofilm. The presence of the bonded glycerol molecules was observed experimentally. Third, the tribodegradation of glycerol molecules produces acids and water within the contact, which was supported experimentally and by MD simulations. It is speculatedm that the synergistic effect of presence of the hydrogen bond network and the water nanofilm is responsible for the drastic friction reduction. Hydrogen network does not allow for water to escape the local contact, while the share is generated in the low viscous water layer resulting in reduced friction. These three steps were found to be crucial in the generation of low friction in the contact.
type of DLC coating was considered and the mechanism of the low friction was proposed. By performing experiments and Molecular Dynamic (MD) simulations, the researchers came up with a model of superlubricity by glycerol, consisting of three points. First, a hydroxylation of the contacting solids is forced by a tribochemical reaction, enhanced by the contact pressure and temperature. Second, the glycerol molecules absorb on the OH-terminated surface (due to hydroxylation) by hydrogen bond and create a tribofilm. The presence of the bonded glycerol molecules was observed experimentally. Third, the tribodegradation of glycerol molecules produces acids and water within the contact, which was supported experimentally and by MD simulations. It is speculatedm that the synergistic effect of presence of the hydrogen bond network and the water nanofilm is responsible for the drastic friction reduction. Hydrogen network does not allow for water to escape the local contact, while the share is generated in the low viscous water layer resulting in reduced friction. These three steps were found to be crucial in the generation of low friction in the contact.
The details of the research can be found in the original article Superlubricity and tribochemistry of polyhydric alcohols, C. Matta, L. Joly-Pottuz, M. I. De Barros Bouchet, J. M. Martin, M. Kano, Qing Zhang, and W. A. Goddard.
Credit for image: reprinted with permission from “Superlubricity and tribochemistry of polyhydric alcohols”. Copyright 2016 American Chemical Society.
Leave a Reply
You must be logged in to post a comment.

Yes, glycerol has certainly been the non-toxic lubricant of choice for “sensitive” applications; it is a common constituent of personal lubricants. Not sure it will prove as useful in industrial applications though, owing to its inherent humectant properties.
From my experience glycerol is not really convenient to use even in the lab, since it intakes water in the open air. I am not sure, if this affects its superlubricity property though.