I am currently working as a Postgraduate Researcher at the University of Leeds, where I am actively involved in research activities. Prior to this, I successfully completed my master's degree through the renowned Erasmus Mundus joint program, specializing in Tribology and Bachelor's degree in Mechanical Engineering from VTU in Belgaum, India. Further I handle the social media pages for Tribonet and I have my youtube channel Tribo Geek.
Friction of Materials
Table of Contents
Introduction
The friction of materials is an important property to be studied to understand its role in any mechanical applications. The tribological properties of any material are determined by its frictional behaviour, this behaviour will determine the life of the materials. Every material has its own behaviour for friction, and it depends on the material properties which need to be understood in order to study its frictional behaviour. This article discusses the frictional properties of metals, ceramics, polymers and solid-lubricated contacts.
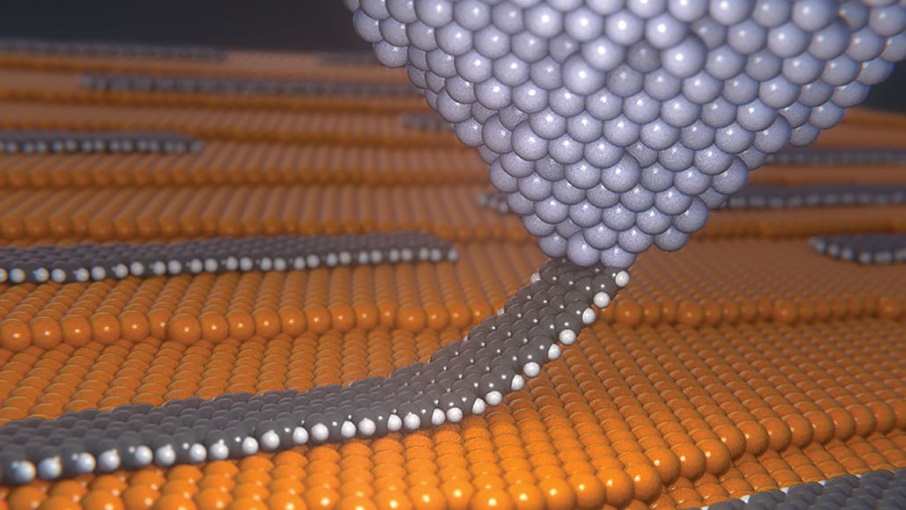
Fig-1 materials that defy friction on the atomic scale [1]
Friction of metals
The friction on metals mainly depends on the surface cleanliness, when the metallic surface is clean then it exhibits high adhesion leading to high friction and wear. The metallic bonds are formed at the interfaces of the two materials in contact leading to the transfer of one metal layer over the other causing the wear debris. The formation of any contamination or any oxide layers can reduce the friction loss at the surface of the metals. There are also various other factors affecting the friction on the metals such as the operating conditions which include the applied loads, temperature, sliding velocity, pressure, gaseous environment, humidity etc [2].
- Effect of load: The formation of the metallic oxide layer on the surface of the material reduces friction. During the operation, if the applied load is high there is the formation of wear debris at the interface which forms the transfer layer. This transfer layer helps in reducing the friction at the surface whereas the low causes elastic deformation breaking the oxide layers and leading to high friction.
- Effect of sliding velocity/ contact pressure: The increase in sliding velocity or contact pressure causes the surface frictional heating leading to a drop of COF as a function of sliding velocity.
- Effect of temperature: The increase in temperature causes the phase transformation from the solid state which degrades the mechanical proper of the materials. Further, the melting point and the strength of materials drop causing increased adhesion on the surface and resulting in high friction. In some cases, the increased temperature also causes a reduction in friction by increasing the rate of oxidation at the interface.

Fig-2 Friction in metallic applications [3]
Friction of ceramics
The friction in ceramics is quite different in comparison to metals because of their bonding nature with respect to their inter-atomic forces with covalent and ionic bonding. They show very limited plastic flow at room temperature and less ductility in comparison to metals. However, they have good mechanical strength, oxidation at elevated temperatures and resistant properties towards corrosive environments. There are properties such as fracture toughness, sliding speed, applied load and temperature which affects the frictional properties of the ceramics [2].
- Effect of fracture toughness: fracture toughness is one of the important properties which affects the friction of ceramics. The increase in fracture toughness reduces the friction of the ceramics. At the full contacts, a fracture occurs that dissipates the energy at the contact interface contributing to the friction.
- Effect of normal load: The increase in normal load causes an increase in friction at the ceramic surface. The increased loads lead to the formation of cracks which in turn causes the increase in friction and wear at the surface.
- Effect of temperature and velocity: The effects of temperature and velocity on the ceramics can be understood in the formation of tribochemical films and the extent of fracture at the surface. The friction coefficients increase with the increase in temperature, this is due to the high interface temperature which leads to the material softening which results in a lower contact area and high friction.

Fig-3 Schematic representation of ceramic bearings [4]
Friction of polymers
The frictional behaviour of the polymers is quite different in comparison to the metals and ceramics. They exhibit very low friction coefficients due to their low elastic modulus and lower strength. They lack rigidity and strength, and they flow at modest pressure and temperatures. Hence the polymer composites are used to improve their frictional properties thereby applying in various applications. The basic forces are responsible to vary the frictional properties of the polymers are adhesion, deformation, and elastic hysteresis. As per the adhesion analysis, it is found that the surface roughness and the normal load affect the friction coefficients in the polymers. There are also factors such as asperity deformation, sliding velocity, and temperature that affect the frictional properties of the polymers.
- Effect of normal load on asperity deformation: The increase in the normal load causes the large asperity deformation which results in a decrease in friction coefficient.
- Effect of sliding velocity and temperature: In polymers, there are various materials that react differently with the sliding velocity and temperatures. In Viscoelastic materials, the increase in temperature causes deformation which is equivalent to a decrease in sliding velocities that affects the frictional behaviours.

Fig-4 Image of polymer gears experiencing friction [5]
Friction in solid lubricated contacts
The most used solid lubricants are graphite and molybdenum disulphide which exhibit lower frictional behaviour the lubrication. The reasons mainly include
- Graphite: In the case of graphite, it has a layer-lattice crystal structure which has the ability to form a strong chemical bonding with gases such as water vapours. This adsorption of the molecules on the surface results in lower friction coefficients. The graphite structure has lamellae that slide parallel to the plane which is low energy surface and causes little adhesion.
- Molybdenum disulphide: In molybdenum disulphide, they have a hexagonal lamellar crystal structure same as the graphite. These layers in the crystal structure are tightly packed and are covalently bonded between the atoms which are very strong. These layers are separated by a large distance which is supported by weak van der Waals forces. Thus the materials are strongly anisotropic with very mechanical and physical properties thus they act as self-lubricants and exhibit better frictional properties.
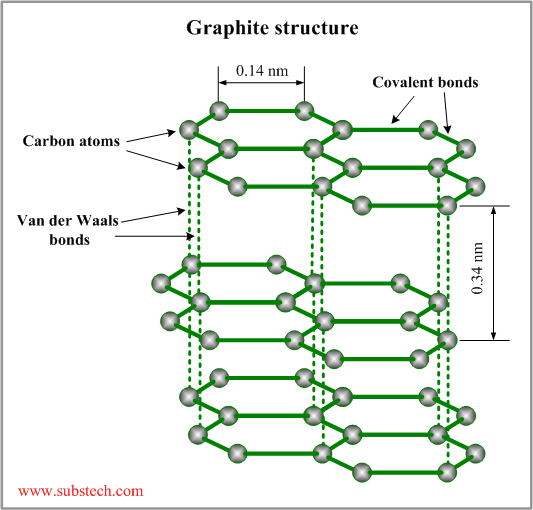
Fig-5 Structure of graphite [6]
Reference
[1] https://www.sciencenews.org/article/scientists-seek-materials-defy-friction-atomic-level
[2] Bhushan, B., 2013. Introduction to tribology. John Wiley & Sons.
[3] https://metalpowdergroup.com/powders-for-friction-applications/
[5]https://www.hardiepolymers.com/knowledge/what-determines-friction-between-thermoplastic-components/
[6] https://www.substech.com/dokuwiki/doku.php?id=graphite_as_solid_lubricant


Be the first to comment