I am currently working as a Postgraduate Researcher at the University of Leeds, where I am actively involved in research activities. Prior to this, I successfully completed my master's degree through the renowned Erasmus Mundus joint program, specializing in Tribology and Bachelor's degree in Mechanical Engineering from VTU in Belgaum, India. Further I handle the social media pages for Tribonet and I have my youtube channel Tribo Geek.
Solid-solid contact
Table of Contents
Introduction
The solid-solid contacts are formed when there is an asperity-to-asperity interaction at the interface of two surfaces. This contact is caused due to the interatomic interactions that lead to adhesion. Further, the adhesion of the surfaces can be considered either physical or chemical in nature, the chemical contacts involve the covalent, ionic, or metallic bonds. The physical contact involves the hydrogen and van der Waals forces which are much weaker compared to the chemical bonds. The solid-solid contact is mainly due to adhesion which depends on the material interface conditions such as crystallographic orientation, crystal structure, the solubility of one material into another, surface cleanliness, applied load, temperature, contact duration, etc.
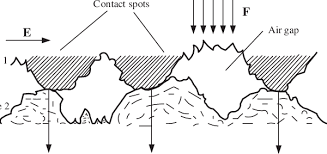
Fig-1 Image showing the solid-solid contact [2]
Type of bonds
There are mainly two types of bonds that interact with the solid-solid contact leading to adhesion, they are physical bonds and chemical bonds. The physical bonds are mainly hydrogen bonds and van der Waals bonds and chemical bonds are covalent, ionic, and metallic bonds.
Covalent bond
Covalent bonds are strong bonds that consist of a pair of electrons that has opposite magnetic spins and are formed due to sharing of electron between two atoms. The covalent bonds formed at the surface interface during the material interaction are the same as it is when formed at the solid medium. In most of the solids with covalent bonds, the covalent bonds need an activation energy to get relaxed. However, the covalent solids are highly elastic materials which makes them more difficult to activate and it is difficult to obtain the larger area of contact at the interface when the joining loads are applied.

Fig-2 Schematic representation of covalent bond [3]
Ionic bond
Ionic bonds are also termed electrostatic bonds and are formed by ionic charges which are when there is the transfer of negative and positive ions between the two atoms. The ionic bonds are formed due to the columbic attractions of the, unlike ions. In the case of metals when there is less attraction in the valence electrons it tends to form ionic bonds with the non-metals. The separation between the two materials when brought close to each other is termed atomic spacing. The adhesion at the interface of the material is electrostatic in nature, the transfer of charges occurs at the contact interface or the separation of the materials. Whenever there is a contract between the conductive and non-conductive materials then the non-conductive materials will become electrically charged due to the friction at the interface leading to ionic bonding.

Fig-3 Schematic representation of ionic bond [4]
Metallic bond
In the case of metallic bonding, there is a layer of electrons called delocalized electrons which are not bound to any particular atoms and are free to drift along the surface. These delocalized electrons tend to form metallic bonds at the surface interface of another material. Whenever the two surfaces are at an atomic distance apart then there is the formation of the metallic bond, in case of the identical metals, there is a large force needed to pull the metals. The adhesive strength at this interface mainly depends on the surface cleanliness, elastic stresses, and surface roughness of the materials.

Fig-4 Schematic representation of metallic bond [5]
Hydrogen bond
A hydrogen bond is a type of physical bond which comparatively weaker than chemical bonds. The hydrogen atom can exist both positively and negatively charged where the positive charge is obtained by the removal of only the electron and the negative charge can be obtained by imperfect shielding of the electron in the nucleus. This negatively charged nature of hydrogen has the tendency of acquiring the electron from the neighboring atom by the ionic attraction which leads to hydrogen bonding. The adhesion in the polymers is mainly due to the hydrogen bonds at certain polar atoms which has the tendency to form hydrogen bonding. The hydrogen bonds are the strongest secondary force of attraction in compared to the other bonds.

Fig-5 Schematic representation of hydrogen bond [6]
Van der Waals bond
These are the weakest bonds compared to the other atomic bonds. It is formed at the atoms and molecules where there is no formation of chemical bonds. In the case of polar molecules, it is formed by dipole-dipole interactions and in the case of non-polar molecules, it is formed due to the fluctuations in the dipoles of the individual atoms. There are studies that show that surface roughness can increase the magnitude of van der waals forces when the interacting surfaces are smooth.

Fig-6 Schematic representation of van der Waals bond [7]
Reference
[1] Bhushan, B., 2013. Introduction to tribology. John Wiley & Sons.
[3] https://www.britannica.com/science/covalent-bond
[4] https://www.britannica.com/science/ionic-bond
[5] https://www.expii.com/t/metallic-bond-formation-compounds-8645
[6] https://www.differencebetween.com/difference-between-hydrogen-bond-donor-and-acceptor/
Be the first to comment