Industrial Engineer with focus on Tribology and Sealing Technology. Team player with an open-minded mentality author of several scientific publications and an industrial patent. Interested in Lean Management, Innovation, Circular Economy, Additive Manufacturing and Connected Objects Technology.
Elastomers
Table of Contents
Glass Transition Temperature
It is not clear what is the difference between an “elastomer” and a “rubber”. It is nowadays accepted to use them indistinctively and this is how it is treated in the following document. To exhibit elastomeric behaviour, a polymer must be flexible and recover from substantial deformation at temperatures above about 0°C. This requires the polymer to be substantially amorphous, and above its glass transition temperature, so that chain segments have adequate mobility to allow the material to return to its original state after the stress is removed.
Whether an amorphous polymer is a thermoplastic, or an elastomer depends on its glass transition temperature (). This is the temperature above which a polymer becomes soft and pliable, and below which it becomes hard and glassy. If an amorphous polymer has a below room temperature, that polymer is an elastomer, because it is soft and rubbery at room temperature. If an amorphous polymer has a above room temperature, this is classified as a thermoplastic, because it is hard and glassy at room temperature. A general rule of thumb is that for amorphous polymers, elastomers have lower glass transition temperatures than the thermoplastics. Keep in mind that this only works for amorphous polymers, and not for crystalline polymers. Vulcanized rubbers do not melt, they decompose.
| Material | (°C) | |
| Acrylonitrile butadiene styrene | ABS | 105 |
| Poly-3-hydroxybutyrate | PHB | 15 |
| Poly(carbonate) | PC | 145 |
| Poly(methyl methacrylate) | PMMA atactic | 105 |
| Poly(vinyl acetate) | PVAc | 30 |
| Poly(vinyl alcohol) | PVA | 85 |
| Poly(vinyl chloride) | (PVC | 80 |
| Polyamide | PA | 47–60 |
| Polychlorotrifluoroethylene | PCTFE | 45 |
| Polyethylene terephthalate | PET | 70 |
| Polylactic acid | PLA | 60–65 |
| Polynorbornene | 215 | |
| Polypropylene | PP atactic | −20 |
| Polypropylene | PP isotactic | 0 |
| Polystyrene | PS | 95 |
| Polysulfone | 185 | |
| Polytetrafluoroethylene | PTFE | 115 |
| Polyvinyl fluoride | PVF | −20 |
| Polyvinylidene fluoride | PVDF | −35 |
| Tire rubber | −70 | |
Classification of Elastomers
The fist classification is upon the elastomer origin:
- Natural
- Synthetic
It is also common to classify them according to the linkage type between their atoms:
- Saturated rubber: a saturated compound is a chemical compound that has a chain of carbon atoms linked together by single bonds. These kinds of rubbers only cannot be cured by sulfur vulcanization. Rubbers with a saturated main chain (possibly with hetero-atoms) present a good resistance to oxygen, temperature, and o-zone.
- Unsaturated rubbers: An unsaturated compound is a chemical compound that contains carbon-carbon double bonds or triple bonds. Rubbers with unsaturation in the main chain (R-rubbers) are more sensitive to o-zone and heat ageing than rubbers which are fully saturated, or which have an unsaturation in a side chain (M-rubbers). This kind can be cured by sulfur vulcanization.
| Unsaturated rubbers | Saturated rubbers |
| Natural rubber (NR) | Polyacrylic rubber (ACM, ABR) |
| Synthetic polyisoprene (IR for isoprene rubber) | Silicone rubber (SI, Q, VMQ) |
| Polybutadiene (BR for butadiene rubber) | Fluorosilicone Rubber (FVMQ) |
| Chloroprene rubber (CR) | Fluoroelastomers (FKM, and FEPM) |
| Butyl rubber (copolymer of isobutylene and isoprene, IIR) | Perfluoroelastomers (FFKM) |
| Styrene-butadiene Rubber (copolymer of styrene and butadiene, SBR) | Ethylene-Propylene(-Diene) Rubber (EPM and EPDM) |
 Figure 1. The most commonly used elastomers in tire compounds are styrene butadiene rubber, natural polyisoprene rubber, natural polyisoprene rubber, butyl rubber, and ethylene propylene rubber.
Figure 1. The most commonly used elastomers in tire compounds are styrene butadiene rubber, natural polyisoprene rubber, natural polyisoprene rubber, butyl rubber, and ethylene propylene rubber.
The main rubber types are listed below:
- Natural rubber (NR)
- Synthetic Polyisopropene (IR)
- Butadiene rubber (BR)
- Styrene-Butadiene Rubber (SBR)
- Ethylene-Propylene(-Diene) Rubber (EPM and EPDM)
- Butyl rubber (IIR)
- Nitrile Rubber (NBR)
- Polychloroprene (CR)
- Fluorocarbon rubber (FKM, FEPM, FFKM)
- Silicone rubber (O)
- Acrylate rubber (ACM)
- Ethylene-Acrylate-Copolymers (EAM)
- Hydrogenated nitrile rubber (HNBR)
Crosslinking of elastomers
Elastomer cure systems are designed to give stable polymeric network. The curing process or vulcanization is a chemical process for converting natural rubber or related polymers into more durable materials by the addition of sulfur or other equivalent curatives or accelerators. These additives modify the polymer by forming cross-links (bridges) between individual polymer chains. Vulcanized materials are less sticky and have superior mechanical properties.
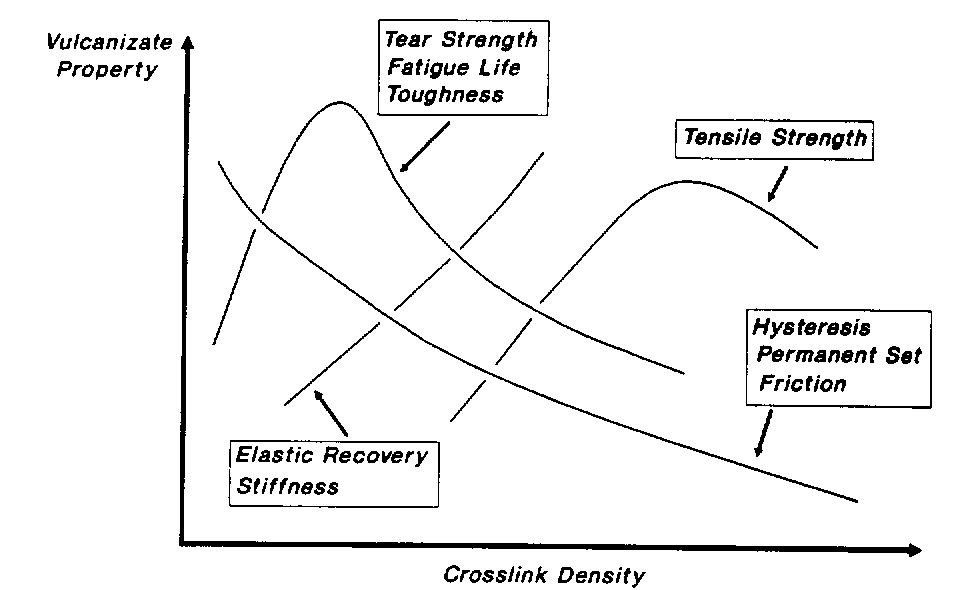
Figure 2. Elastomer properties in function of its crosslink density.
Nowadays 90% of the rubber produced is cured with sulfur, around 6% use peroxide vulcanization and the rest need from other specific curing methods. The number of allylic hydrogens gives an idea of the ease to vulcanize.
Vulcameters are used to measure the change in stiffness while the cross links due to vulcanization are formed. The degree of vulcanization is measured on the basic principles of rubber elasticity theory.
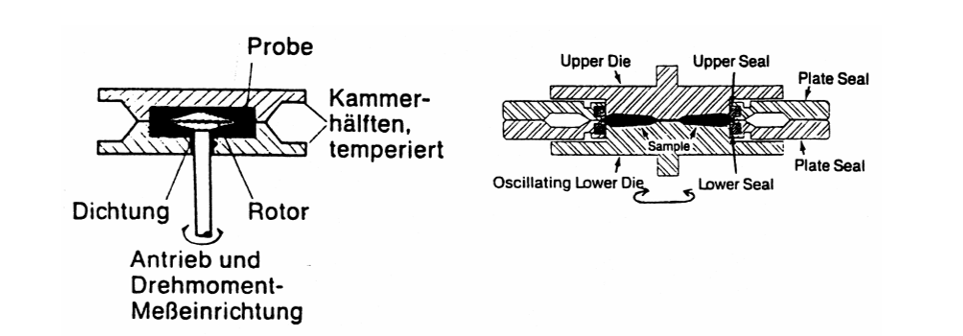
Figure 3. Vulcameter with and without a rotor.
The following “vulcanization curve” shows the rubber stiffness variation in time. The oven temperature is generally kept constant during vulcanization.
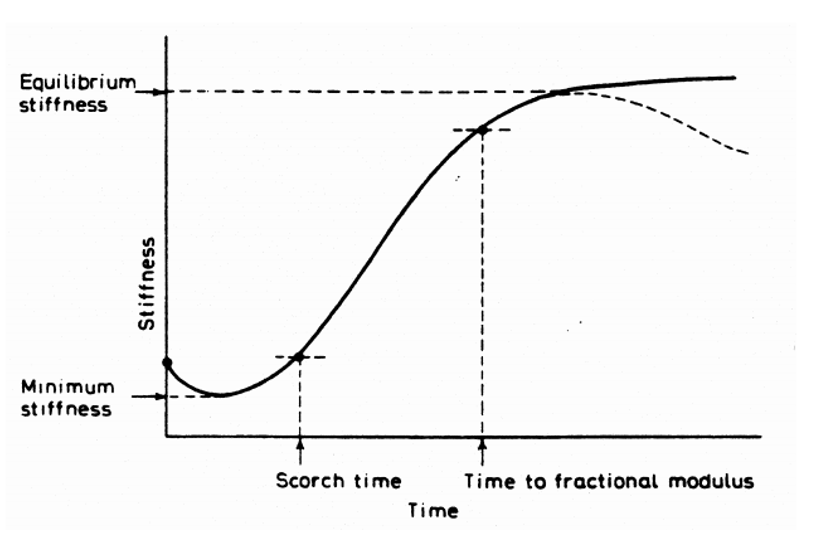
Figure 4. Typical curing/vulcanization curve or rheogram.
In such a plot, also called a rheogram, the following characteristic parameters are often given:
Vulcanization curve characteristics |
|
| tS2 | The scorch time: the time needed to increase the torque by two units above the minimum value. The vulcanization has just started at this time. Within the scorch time, the rubber compound can still be processed (processing time). |
| ML | Minimum torque: Determined by the heating of the compound and the beginning of vulcanization |
| MH | Maximum torque: Determined by the extent of crosslinking |
| t50 | The time needed to bridge 50 % of the difference between minimum and maximum torque. |
| t90 | The time needed to bridge 90% of the difference between minimum and maximum torque. This time is generally used as a typical value for an optimal vulcanization time. |
The way the curve develops after full vulcanization is from high importance and any of the following three options can occur:
- Marching: the cure keeps increasing the stiffness.
- Stable: levels off to reach an equilibrium plateau.
- Reversion: decrease of torque after reaching the maximum stiffness.
Reversion is common with highly unsaturated rubbers. It is a nasty phenomenon, which is the result of preliminary degradation or oxidation of the newly formed sulfur bridges. Reversion partly undoes the effect of the vulcanization. The recording of a vulcameter or rheometer-curve is always an important step in the preparation of a rubber compound. With the aid of this curve, it can be seen whether the vulcanization recipe is properly adjusted to the temperature profiles belonging to the process technique. At low temperatures, the compound must remain mouldable (process-able) for a long enough time, while at the vulcanization temperature a dense enough network must be formed as quickly as possible.
Examples of sulfur vulcanization recipes:
| Recipe | Time | Temperature (°C) | Result |
| NR + 6 phr Sulfur | 4 hours | 140 | little vulcanization |
| NR + 6 phr Sulfur + 5 phr ZnO | 4 hours | 140 | some higher yield |
| NR + 2 phr Sulfur + 5 phr ZnO + 1 phr accelerator | 20 minutes | 140 | fully vulcanized |
Many elastomeric products require from a post-curing process. This step in usually made out from the mould. The piece is introduced in a high temperature oven for a specific amount of time. This process helps to create the final crosslinks. Post-curing also contributes to locking of the polymeric chains so the material tendency to come back to its original shape (memory) is reduced.



Be the first to comment