Industrial Engineer with focus on Tribology and Sealing Technology. Team player with an open-minded mentality author of several scientific publications and an industrial patent. Interested in Lean Management, Innovation, Circular Economy, Additive Manufacturing and Connected Objects Technology.
Fluoroelastomers
Fluorocarbon elastomers are a rubber specialty. These are well-known for having the best temperature resistance and for being highly inert. Fluoroelastomers also show a high resistance against mineral oil-based lubricants. The chemical resistance and high temperature stability is due to the bulkiness of the fluorine atoms and the high energy Carbon-Fluor bond. Fluoroelastomers are saturated rubbers and often need to be cured by bisphenol (and peroxides), instead of sulfur, vulcanization.
Fluor atoms are relatively big, and it has one of the highest molecular bonding energies.
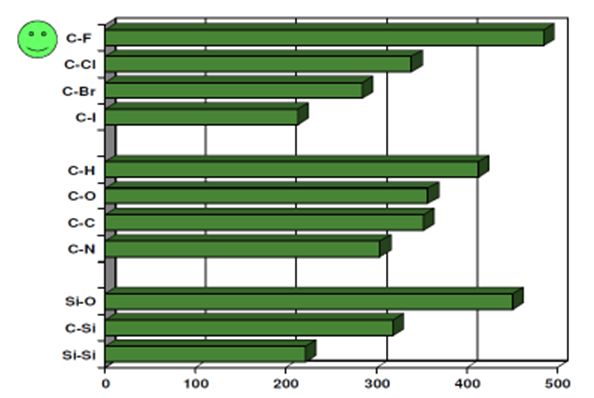
Average single bonds energies (kJ/mol).
Hydrocarbon rubbers (hydrogen and carbon) are classified as nonpolar, however, the substitution with fluorine leads to polar molecules. It is apparent that bond dissociation energies establish basic parameters for both elastomer thermal stability and ease of abstraction for crosslink site development.
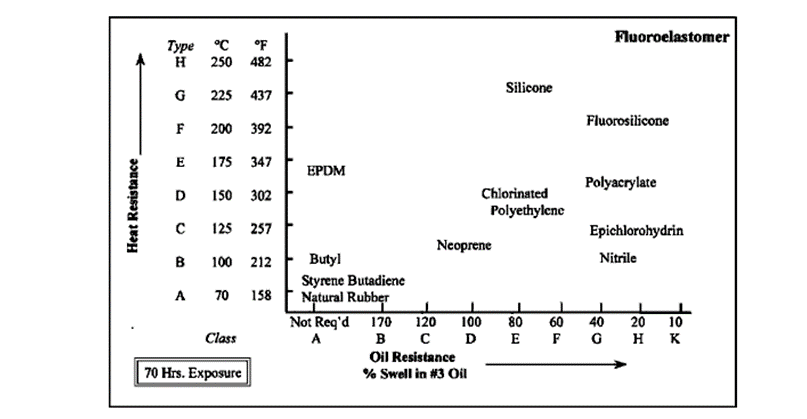
Heat and oil resistance of elastomers (ASTM).
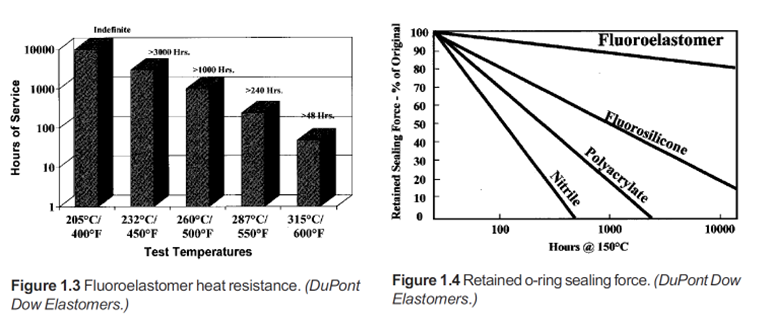
Generally, fluorocarbon chains are relatively stiff compared to hydrocarbons, so fluoroelastomers exhibit rather slow relaxation and recovery from strain (i.e., leathery rather than highly resilient behavior). Thus, most fluoroelastomers are used in static, rather than dynamic, applications.
The main characteristics of fluoroelastomers are:
- Excellent temperature resistance
- High chemical stability
- Good weather, ageing and oxygen stability
- Excellent resistance to mineral oils and fats
- Low gas permeability
- Very good resistance in non-polar media
- Temperature range from -40°C to 225°C
FPM is an international abbreviation according to DIN/ISO, whereas FKM is the short form the fluoroelastomers category according to the American standard ASTM. Viton® is the registered trademark of DuPont Performance Elastomers, as is Tecnoflon® for Solvay Speciality Polymers, DyneonTM for 3M Dyneon and DAI-ELTM for fluoroelastomers supplied by Daikin.
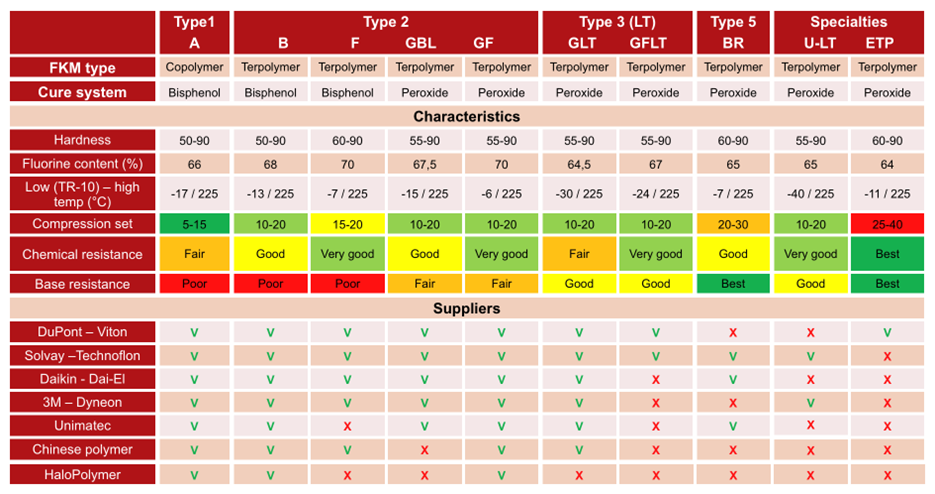
Fluoroelastomer groups, properties, and suppliers.
Monomers for FKM
Elastomers are compounds of different monomers. Each monomer has its own proprieties and the amount of each one determines the specific set of proprieties of the elastomer. The combinations of monomers used must produce substantially amorphous copolymers with low enough glass transition temperatures for having an elastomeric behavior at the application temperature.
The following are the main monomers used in FKM elastomers.
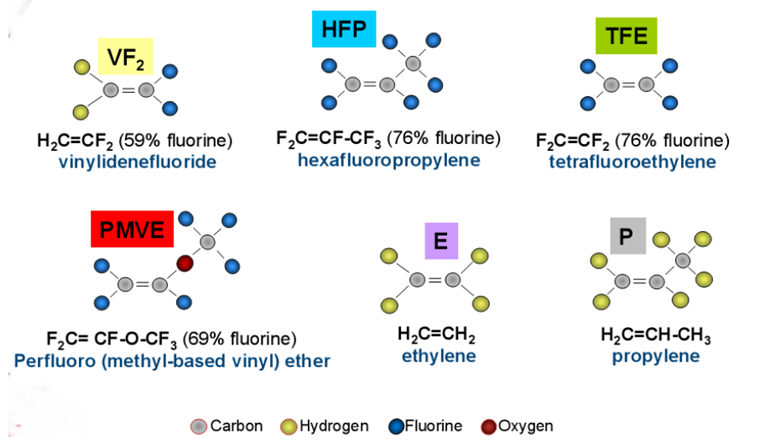
Monomers for FKM production (source: Polycomp).
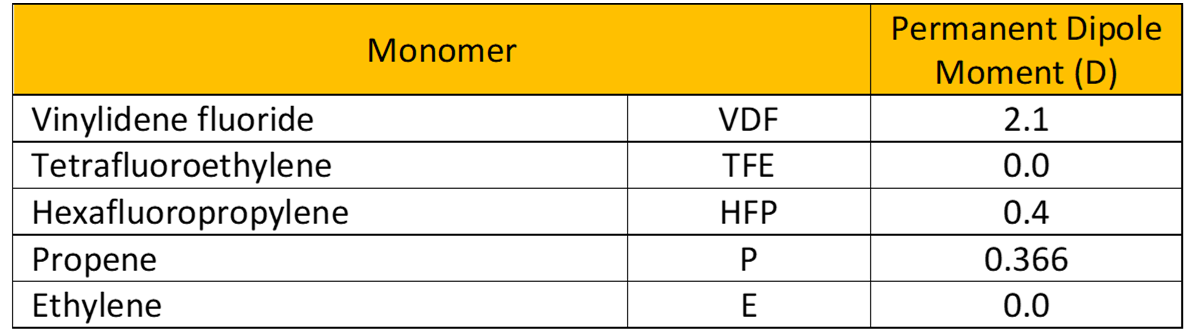
Permanent dipole moment of the FKM building monomers.
The major family of VDF/HFP and VDF/HFP/TFE fluoroelastomers can be cured readily with bisphenols without incorporation of special Cure-Site-Monomers (CSM). However, highly fluorinated polymers require from specific monomer sequences present in small amounts to provide active sites for crosslinking.
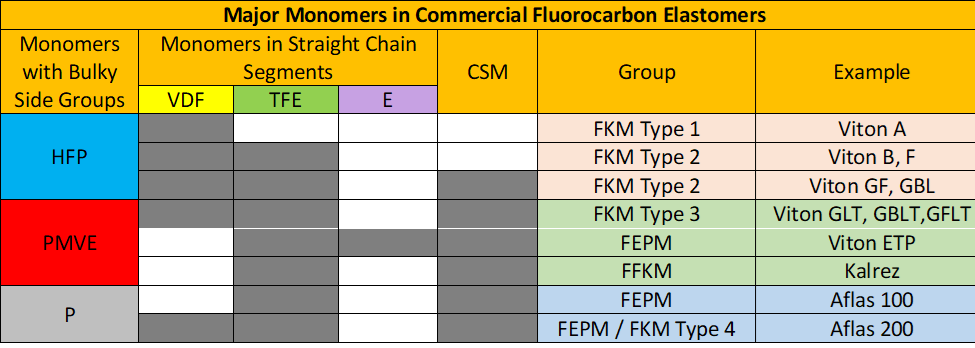
Classification
Fluorocarbon elastomers are typically classified in three groups:
- FKM: VDF copolymers
- FEPM: TFE/Olefin copolymers
- FFKM: perfluoroelastomers
Most rubber compounds based on high performance elastomers require a “post-cure” after their normal press cure to achieve optimal cured physical properties, so do FKMs. The post-cure step is usually performed in an air-circulating oven (at ambient pressure) for 2 to 24 hours at 150-250 °C, depending on the compound. Post-cure step is also used to eliminates residual volatiles formed during the vulcanization process that might disrupt or destroy the chemical crosslinks formed during curing.
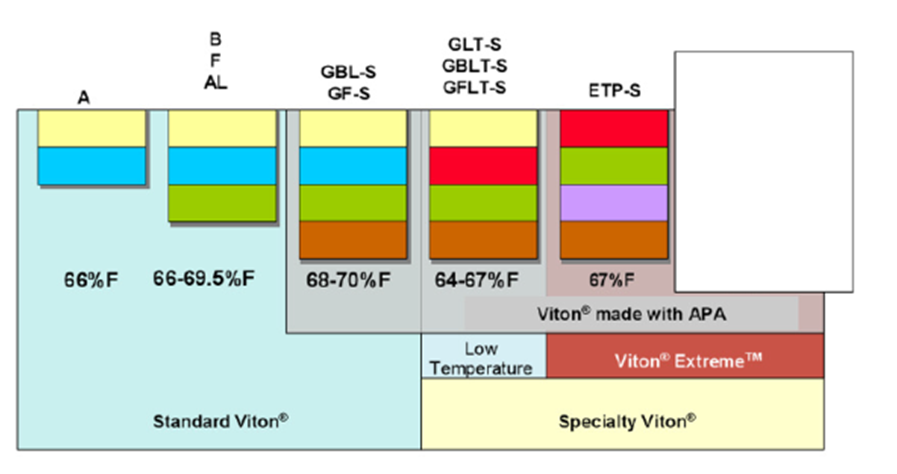
DuPont® elastomers building blocks (source: Polycomp).
It is difficult to give a price indication, since this in great part depends on the size and production volumes. The following table might give an idea:
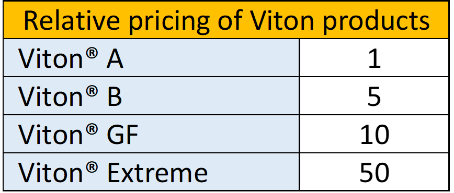
Fluoroelastomers. (Source:ERIKS)
References
- Jiri George Drobny, “Fluoroelastomers Handbook (Second Edition)”, William Andrew Publishing, 2016, ISBN 9780323394802, https://doi.org/10.1016/B978-0-323-39480-2.00020-8.
- J. Carrell, R. Lewis, and T. Slatter, “The Interaction of Biolubricants with Elastomers , Tested Through Hanson Solubility Parameter and Stress Relaxation Tests,” in The 17th Nordic. Symposium on Tribology, 2016, pp. 1–10.
- Polycomp website (https://www.polycomp.nl/)

Be the first to comment