I am currently working as a Postgraduate Researcher at the University of Leeds, where I am actively involved in research activities. Prior to this, I successfully completed my master's degree through the renowned Erasmus Mundus joint program, specializing in Tribology and Bachelor's degree in Mechanical Engineering from VTU in Belgaum, India. Further I handle the social media pages for Tribonet and I have my youtube channel Tribo Geek.
Infrared spectroscopy
Table of Contents
Introduction
Infrared (IR) spectroscopy deals with the infrared region of the electromagnetic spectrum, i.e., light having a longer wavelength and a lower frequency than visible light. Infrared light was discovered in the 19th century which led to the use of infrared lights in various applications.
Definition
Infrared Spectroscopy is a technique for analyzing the interaction of molecules with infrared light. The range of Infrared region is 12800 ~ 10 cm-1 and can be divided into near-infrared region (12800 ~ 4000 cm-1), mid-infrared region (4000 ~ 200 cm-1) and far-infrared region (50 ~ 1000 cm-1). The concept of IR spectroscopy can be generally analyzed in three different ways first one by measuring reflection, second by emission, and third by absorption. The major use of infrared spectroscopy is to determine the functional groups of molecules, relevant to both organic and inorganic chemistry An IR spectrum is essentially a graph plotted with the frequency or wavelength on the X-axis and infrared light absorbed on the Y-axis. An illustration highlighting the different regions that light can be classified into is shown in Fig-1. IR Spectroscopy detects frequencies of infrared light that are absorbed by a molecule and this absorption takes place due to the ability of molecular bonds that are corresponding to the specific frequency [1].
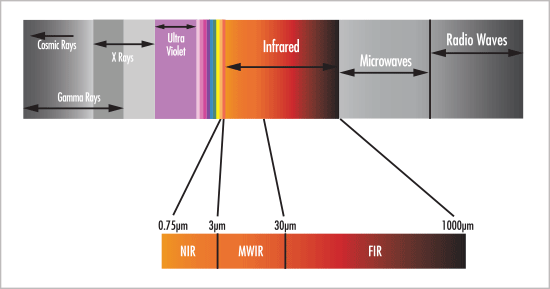
Fig-1 IR Spectrum [2]
Infrared absorption spectroscopy
Infrared absorption spectroscopy is the method to determine the structures of molecules with the molecule’s characteristic absorption of infrared radiation. The infrared spectrum is nothing but a molecular vibrational spectrum that when exposed to infrared radiation; sample molecules selectively absorb radiation of specific wavelengths causing a change in the dipole moment of sample molecules. Consequently, the vibrational energy levels of sample molecules are transferred from the ground state to the excited state. The frequency of the absorption peak in the molecules is determined by the vibrational energy gap. The number of absorption peaks is related to the number of vibrational freedoms of the molecule and the intensity of absorption peaks is related to the change of dipole moment corresponding to the possibility of the transition of energy levels [3]. Therefore, analyzing the infrared spectrum helps in obtaining the various structural information of a molecule. The different IR absorption spectrometer setups are shown in the Fig-2.

Fig-2 Absorption IR spectrometer setups [4]
Dispersive IR Spectrometers
Dispersive IR Spectrometers are used to generate the spectra by dispersing the incoming radiations into frequency or spectral components. The common examples of dispersive elements are gratings and prisms that disperse the incoming spectrum of radiation. There are mainly two types of dispersive spectrometers; they are monochromators and spectrographs. The major difference between these two spectrometers is the case monochromators use a single detector, a narrow slit, and a rotating dispersive element that allows the user to observe the selected wavelengths. Whereas spectrographs use an array of detector elements and a stationary dispersive element which helps in obtaining a wide range of wavelengths at the same time. The schematic image of the monochromator dispersive spectrometer is shown in the Fig-3.

Fig-3 Schematic image of the monochromator dispersive spectrometer [5]
Components of IR spectrometers
The basic components of an IR spectrometer include a radiation source, monochromator, and detector. The schematic representation of the IR spectrometer with its components is shown in Fig-4.
IR radiation source: The commonly used IR radiation sources are inert solids that are heated electrically to promote the thermal emission of radiation in the infrared region of the electromagnetic spectrum.
Monochromator: The monochromator is a device that is used to disperse or separate a broad spectrum of IR radiation into individual narrow IR frequencies. After the incident radiation travels through the sample species, the emitted wavefront of radiation is dispersed by a monochromator (gratings and slits) into its component frequencies. A combination of prisms or gratings with variable-slit mechanisms, mirrors, and filters comprise the dispersive system. Narrower slits give better resolution by distinguishing more closely spaced frequencies of radiation and wider slits allow more light to reach the detector and provide better system sensitivity. The emitted wavefront beam (analog spectral output) hits the detector and generates an electrical signal as a response.
Detectors: Detectors are devices that are used to convert the analog spectral output into an electrical signal. These electrical signals are further processed by the computer using a mathematical algorithm into a final spectrum. The detectors that are used in IR spectrometers can be classified as either photon/quantum detectors or thermal detectors. It is the absorption of IR radiation by the sample, producing a change in IR radiation intensity, which gets detected as an off-null signal (e.g. different from the reference signal). This change is translated into the recorder response through the actions of synchronous motors. Each frequency that passes through the sample is measured individually by the detector which consequently slows the process of scanning the entire IR region.

Fig-4 Dispersive IR spectrometer [6]
Reference:
[1] Nancy Birkner (UCD), and Qian Wang (UCD) on chem.libretexts.org
[3] B. C. Smith, Fundamentals of Fourier Transform Infrared Spectroscopy, CRC press, 1996
[5] https://spie.org/publications/tt61_121_dispersive_spectrometers?SSO=1
[6] https://www.chemicool.com/definition/fourier_transform_infrared_spectrometer_ftir.html

Be the first to comment