I am currently working as a Postgraduate Researcher at the University of Leeds, where I am actively involved in research activities. Prior to this, I successfully completed my master's degree through the renowned Erasmus Mundus joint program, specializing in Tribology and Bachelor's degree in Mechanical Engineering from VTU in Belgaum, India. Further I handle the social media pages for Tribonet and I have my youtube channel Tribo Geek.
Wettability
Table of Contents
Introduction
Wetting is a very important property to understand the bonding and adherence strength of a solid surface or any two materials. The adhesive forces in the liquid help in spreading across a solid surface whereas the cohesive forces in the liquid causes to avoid the liquid from spreading on a solid surface. Hence it is important to understand the hydrophobicity or hydrophilicity of the solid surface which can be measured using the contact angle measurement.
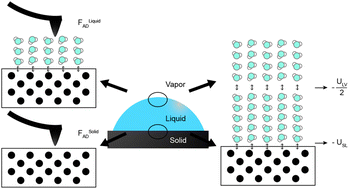
Fig-1 The schematic representation of wettability on a surface [1]
Definition
Wettability is the measurement of the ability of the liquid to interact with other fluids or solids and it is also the measure of the liquid contact angle. Wettability is controlled by balancing the intermolecular interactions of adhesive and cohesive type. The surface forces that are responsible for wetting also affect the capillary effects of the liquid.
Types of wetting
There are two types of wetting, non-reactive wetting, and reactive wetting.
- Non-reactive wetting: If the wetting of liquid on the solid surface proceeded by spreading the liquid on the surface without any interaction or absorption between the interface then it is termed as non-reactive wetting or inert wetting.
- Reactive wetting: If the wetting process between the liquid and the solid medium is where there is influence or reaction between the liquid on the solid medium then it is termed as reactive wetting.
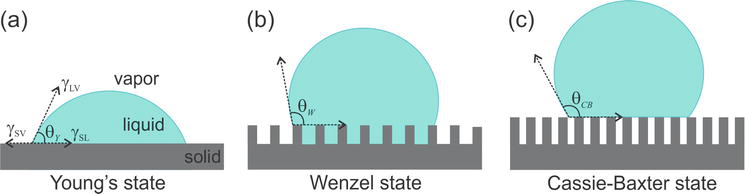
Fig-2 The schematic representation types of wettability [2]
Interaction of wetting on different surfaces
The type of solid surface on which the liquid interacts and spreads over it causes different wettability hence it is important to understand the type of solid surface it interacts. There are mainly two types of solid surfaces interacting with the liquid. They are high-energy and low-energy solid surfaces [3].
- High energy solid surface: Solid materials such as metals, ceramics, glasses, etc which have very strong chemical bonds such as covalent and ionic bonding are very strong. When there is an interaction of liquid on these surfaces then there is a need for larger forces to break the bonding and the liquids attain complete wetting on these surfaces.
- Low energy solid surface: The solids with weaker molecular bonding such as hydrocarbons or fluorocarbons where their molecules are bonded by van der Waals forces or hydrogen bonding etc are termed as low energy solid surfaces. The wetting on these surfaces depends on the type of liquids and it may be partial wetting or complete wetting.
Measuring the wettability
The contact angle is one of the qualitative ways of measuring the wettability of the liquids and it determines if the solid surface is favorable or unfavorable to wetting. If the contact angle is lesser than 90 degrees then it is called the low contact angle which indicated that the surface is favorable for wetting. If the contact angle is lesser than 90degree then it is called high contact angle indicating the surface is unfavorable to wetting. In the case of water, this interaction on the solid surface can be termed as the hydrophilic and hydrophobic nature of the material surface.
- Hydrophilic surface: The surface is called the hydrophilic surface if it attracts the water, in which the water drop spreads on the surface. The wettability of the surface is very good with a low contact angle. The adhesive force between the liquid and solid is high in this case.
- Hydrophobic surface: The surface is called the hydrophobic surface if it repels the water, in this case, the water does not spread over the surface. The wettability of the surface is considered to be not good with high contact angle. The cohesive force is dominating this liquid-solid interaction.

Fig-3 The schematic representation of contact angle and wettable surfaces [4]
Research on wettability
Wearability is one of the important surface properties of the materials, understanding this property helps in applying the materials in various applications. There are various applications of wettability in tribology and in a study on wettability and its effect on oil recovery Norman R Morrow studied its importance in oil recovery under boundary conditions [5]. Earle C Donaldson et.al, studied the methods to determine wettability and its effects on recovery efficiency [6]. In a study, Limping Wen et.al., have reviewed the types of super wettability which includes three-dimensional, two- and one-dimensional surfaces, and their applications in various fields and the problems faced due to this in the fields of energy, resources, environment, and health [7].
Reference
[3] Schrader, M.E; Loeb, G.I. (1992). Modern Approaches to Wettability. Theory and Applications. New York: Plenum Press. ISBN 978-0-306-43985-8.
[4]https://www.igvp.uni-stuttgart.de/en/research/plasma-technology/processes/surface-wettability/


Be the first to comment