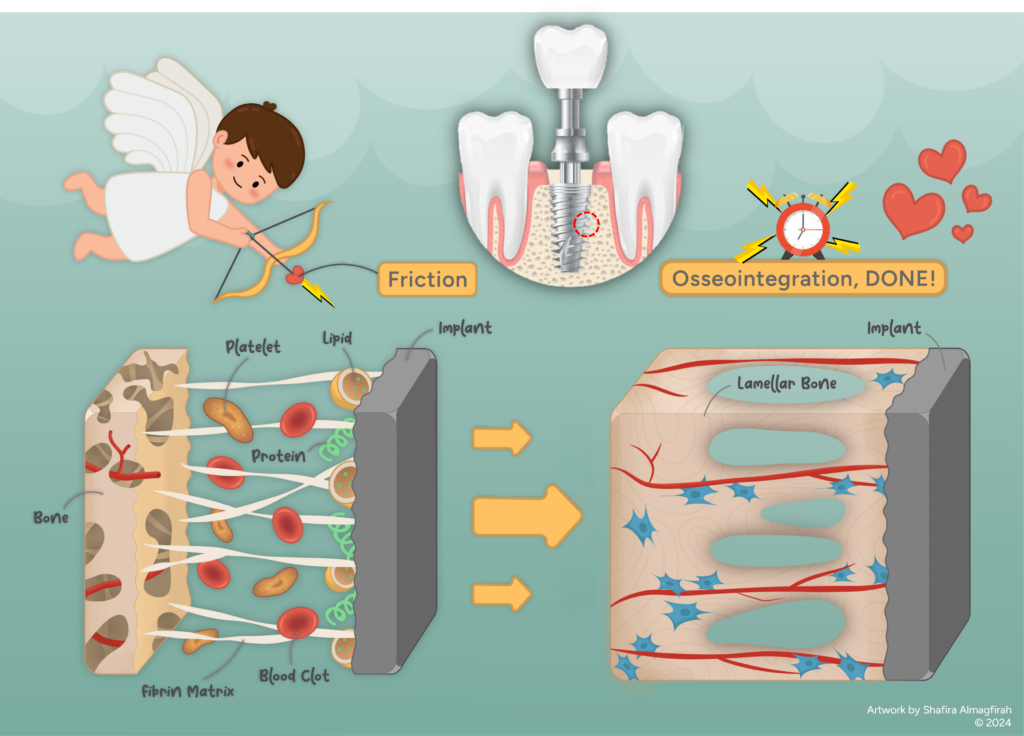
Friction, a “cupid” in implant and host bone relationship
Table of Contents
Introduction
Welcome to the fascinating world of Biotribology, an emerging field exploring friction, wear and lubrication in biological environments, particularly in the context of biocompatible interactions with implantable devices. This article highlighted into the unique role of tribology in the human body, acting as a matchmaker that brings two unlikely friends become one. Imagine this: an implant, like a newcomer, enters a patient’s body, needing time to fit in. Similar to a foreigner in a new country requiring a guide, language-learning and an adjustment period, an implant meets a parallel experience in the human body, a stranger introduced to the local citizens – the bone tissues. Immediate acceptance is not guaranteed, and any misstep can disrupt stability and functionality. This is when the implant needs a “cupid” – something to make the bone tissues “fall in love,” creating a strong bond since their first interaction. In medical terms, this process is osseointegration, where the tribological aspect, specifically friction, plays a crucial role in bringing the implant and bone tissue together.
Osseointegration
Osseointegration is described as a healing process over time, where an implanted device forms a structural and functional connection with living bone tissues [1]. To put it simply, this is when bone cells grow onto the implant surface. A successful integration results in firm fixation, providing stability to the implant during regular use. The discovery of osseointegration dates back to 1952 when Professor Per Ingvar Branemark observed that titanium oculars, implanted into the femurs of rabbits for bone healing studies, could not be removed after fusing with the bone. In 1965, he achieved a significant milestone by successfully inserting a dental implant made of titanium into the upper and lower jawbone, serving as anchor points for his first patient. Osseointegration has proven successful for decades, involving various biomaterials like titanium for dental, knee and hip implants, and polymers for spine and musculoskeletal implants.
The bone healing process is triggered by any lesion, injury, or diseased spot clearly separated from the surrounding healthy tissue in the existing bone matrix [2]. Exposure of the matrix to bodily fluids releases non-collagenous proteins and cell growth factors, activating bone repair. This process involves stages such as woven bone formation incorporation, bone mass and structure adapting to load during osseointegration [3]. These make the osseointegration process widely recognized as a complex phenomenon influenced by numerous factors affecting bone formation. The robustness of this bond hinges on the adhesion of bone tissue cells to the implant surface [4]. The cells must adhere to the implant for osseointegration. Their survival and multiplication while spreading out on the surface are crucial. If they fail to do so, proliferation would not occur, potentially leading to the rejection of the implant by the body. This underscores the critical role of engineers, who need to actively find innovative solutions to address issues at the bone-implant interface and overcome current limitations. The aim is to ensure that all implant biomaterials are well-received and accepted by the local bone environment during that critical time.
Friction roles
Clinicians aim for immediate stability during operations, utilizing biocompatible or cemented components. However, ensuring long-term stability for sustained bone cell growth post-implantation is a challenge. Friction plays a crucial role in surface treatments. Implant designs often incorporate threading screws for mechanical interlocking with the surrounding bone. Additionally, roughened surface coatings facilitate a steady interface connection through friction between implant and bone surfaces. This case can be seen in components like acetabular cups and femoral stems for total hip replacement, tibial trays for knee replacement and spinal implant cages.
Recent findings emphasize factors influencing bone cell behavior on implant surfaces, including physico-chemical characteristics (charge, wettability and specific functional groups) and surface topography (roughness and waviness). Surface treatments aim to achieve higher roughness and interface asperities, ultimately increasing surface friction. Higher friction theoretically means a greater resistant force, signifying improved implant stability [5]. Once locked in place by “friction stability,” the bone healing process activates. Surface roughness and topography accommodate bone cells for adhesion, survival and multiplication onto the implant for osseointegration. This process involves proteins adsorbing to the implant surface, forming an extracellular matrix (ECM) that provides an environment for cell migration and differentiation. ECM synthesis, as well as cell adhesion and proliferation, is significantly influenced by implant surface topography, ranging from nano-roughness to micro-roughness [4].
Extensive efforts aim to enhance bonding between implant biomaterials and host bone for optimal osseointegration. Common methods involve surface modification through physical and chemical treatments, coatings and composites [5, 6]. Plasma ion implantation, a common physical technique, exhibits compatibility with implant surfaces. It reduces contact angles, enhances wettability [7], boosts biomaterial bioactivity, cell adhesion and osseointegration [8, 9]. Other methods like accelerated neutron atom beam, photodynamic therapy, laser radiation and sandblasting show promise in the physical treatment category [10-12]. However, some methods primarily improve interlocking without enhancing hydrophilicity and antimicrobial properties in the biomaterial.
Closure
Osseointegration is crucial for the success of materials like titanium and polymers in different implants. The challenge lies in keeping these implants stable, and engineers are working on choosing the right biomaterials and surface treatments. The main aim is to increase friction through surface treatments, helping bone cells stick to the implant for enough time so they can heal properly. Just think, without friction, like a “cupid” in the first meeting between the implant and bone, they would not have anything to grab onto. This means the local tissues could not grow for recovery, leading to implant failure.
Acknowledgment
The artwork was illustrated by S. Almagfirah (email) based on an idea by M. Taufiqurrakhman.
References
2. Schenk, R.K. and D.J.P. Buser, Osseointegration: a reality. 1998. 17(1): p. 22-35.



Be the first to comment