I am currently working as a Postgraduate Researcher at the University of Leeds, where I am actively involved in research activities. Prior to this, I successfully completed my master's degree through the renowned Erasmus Mundus joint program, specializing in Tribology and Bachelor's degree in Mechanical Engineering from VTU in Belgaum, India. Further I handle the social media pages for Tribonet and I have my youtube channel Tribo Geek.
Adhesion: Definition, Theory and Types
Table of Contents
Introduction
Adhesion is one of the important material behavior to be considered to understand the influence of the interactions between the different materials in nature. This knowledge is also applied in case of the man-made materials for different applications. Adhesive bonding is another important area in material science research that focuses on the creation of new materials or composites.
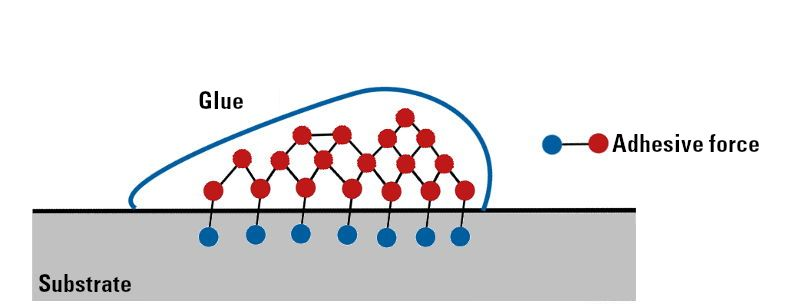
Fig-1 Schematic representation of the adhesion of glue on the surface [1]
Definition
Adhesion is nothing but an attraction between the two dissimilar phases of materials. There are various theories to explain adhesion and it is also considered that adhesion occurs due to various factors such as mechanical interlocking, physical bonding, and chemical bonding, etc. there are various forces responsible for intermolecular interactions such as weak van-der Waals forces, covalent bonds, and due to dipole-dipole interactions, etc [2].
Factors affecting adhesion
The various factors that affect the adhesion are [3]:
- Surface cleanliness: Clean surfaces tends to have more adhesion because in case of the unclean surfaces the presence of surface oxides and absorbed gases might not favor the proper adhesion. Hence the surface is ideally cleaned to lose the matter.
- Physical factors: various physical factors such as acceptance regime time, temperature, and pressure also play a vital role in determining the adhesion on the surface hence the rate of adhesion depends on these factors.
- Contact angle: Contact angle is the measure of the wettability of the surface and hence the surface is more wettable with the lower contact angles. This favors adhesion on the surface.
Types of adhesion theories
1. Mechanical Adhesion: This type of adhesion takes place due to mechanical interlocking between two dissimilar phases which attach to one another by mechanical forces only. It can be usually found in polymers, when the adhering polymer flows into the voids of the substrate, causing an interlock like pieces of puzzles, hardening the liquid causes a strong mechanical bond. The mechanical adhesion is shown in Fig-2.
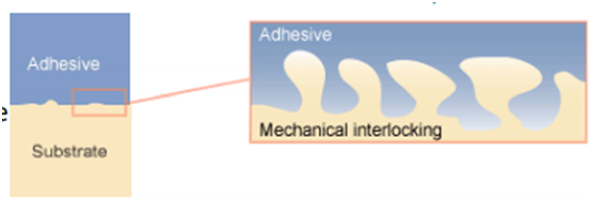
Fig-2 Representation of the mechanical adhesion [4]
2. Electrostatic Adhesion: This type occurs due to an electrostatically charged double bond developed at the bond interface due to the interaction of the adhesive and adherent which contributes significantly to the bond strength. This creates an attractive electrostatic or Coulomb force between the two materials like the two plates of a capacitor. The electrostatic adhesion is shown in Fig-3.
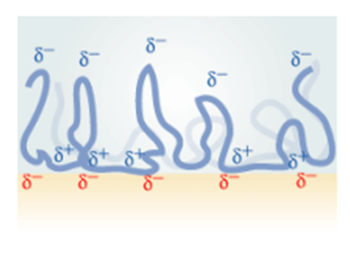
Fig-3 Representation of the Electrostatic adhesion [4]
3. Chemical Adhesion: This is a type of adsorption in those materials with stronger chemical bonds (ionic, covalent metallic) form across the joint interface. Chemical adhesion is a sub-type of specific adhesion. The atoms/molecules of the two adhering materials form chemical bonds that can be of ionic or covalent character. This is usually the strongest form of adhesion. The chemical adhesion is shown in Fig-4.
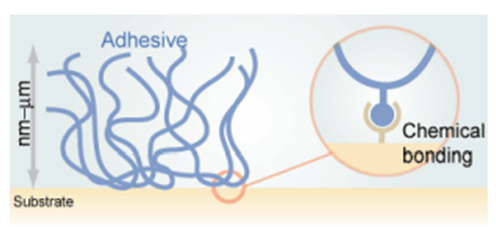
Fig-4 Representation of the Chemical adhesion [4]
4. Dispersive adhesion: It is a type of adhesion that attributes the attractive forces between two materials to intermolecular interactions between molecules of each material. The dispersive adhesion mechanism is widely viewed as the most important of the five mechanisms of adhesion due to its presence in every type of adhesive system and its relative strength. The dispersive adhesion is shown in Fig-5.
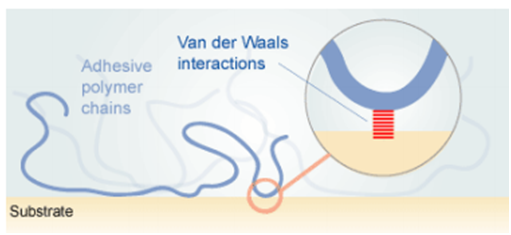
Fig-5 Representation of the dispersive adhesion [4]
5. Diffusive adhesion: Merging of materials through diffusion – when the molecules of both materials are mobile and soluble in each other. Mechanical adhesion on the molecular level: when molecules from one surface can penetrate the counter-surface while still being bound to the phase of their surface of origin (polymer-polymer). The diffusive adhesion is shown in Fig-6.
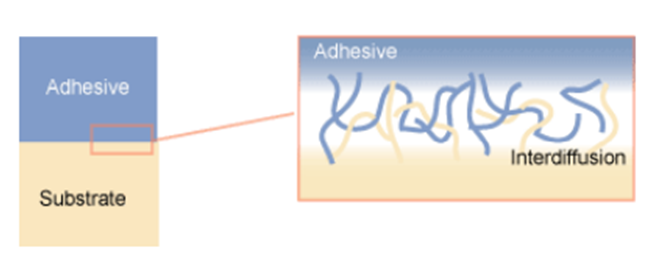
Fig-6 Representation of the diffusive adhesion [4]
Further Reading:
Reference
[1] https://www.buehnen.de/en/service-en/glossary/adhesion
[2] Packham, D.E., 2011. Theories of fundamental adhesion. Handbook of adhesion technology, pp.9-38.
[3] https://roymech.org/Useful_Tables/Adhesives/Adhesive_Bond.html


Be the first to comment