The article is written by Riya Veluri, an editorial team member of Industrial Lubricants. After her graduation, Riya works as a website developer & SEO specialist in Lubrication & Tribology Industry & writes technical articles on Lubricants, Lubrication, Reliability & sustainability.
Dry Lubricants
Table of Contents
What are Dry Lubricants?
Dry lubricants or solid lubricants are substances that, even though they’re within the solid phase, can decrease the friction of two materials that slide against one another without the requirement of any liquid oil medium.
The two most critical dry lubricants comprise are graphite and molybdenum disulfide. They provide good lubrication at high temperatures that oils and liquid-based lubricants cannot work. The low-friction properties of most dry lubricants can be attributed to a layering structure on the molecular level, with weak bonds between layers. The formed layers can slide between each other without much force applied, which gives them low friction characteristics. Dry lubricants can operate at up to 350 degrees Celsius in oxidizing environments and in non-oxidizing conditions solid lubricants like molybdenum disulfide can operate as high as 1100 degrees Celsius.
Four most frequently used solid lubricants
Graphite:
Graphite can be described as one of the three forms of chemical elements.
- Carbon
- Diamond
- Amorphous Carbon
Graphite crystallizes in the hexagonal system. The carbon atoms are strongly bonded together in sheets. Because the bonds between the sheets are weak, Graphite has lower shearing strengths under frictional force. This makes it suitable as a solid lubricant and has been regarded as one of the traditional and the most widely used solid lubrication substances.
It is used in air compressors, the food industry, railway tracks, joints, brass instruments, valves, open gears, ball bearings, and different machine-shop works. It is also used to lubricate locks because a liquid lubricant can allow particles to be stuck in the lock, which can cause problems. It is typically utilized to lubricate internal moving components of firearms in sandy conditions.
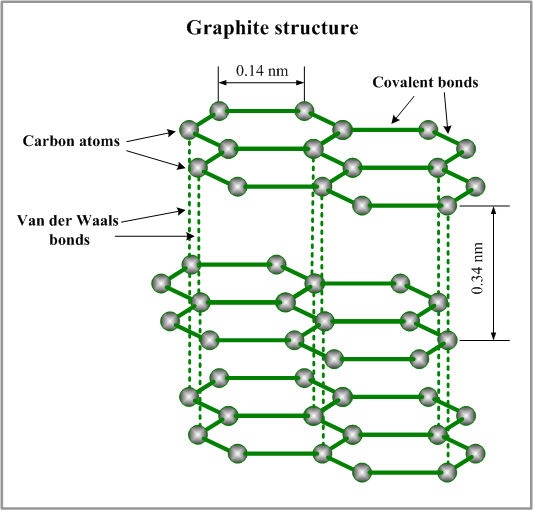
Figure: Graphite Structure
The advantages of graphite solids comprise:
- Good lubrication even in high humidity.
- Protection against fretting corrosion.
- Stability in high-temperature conditions.
- A low coefficient of friction when under high load conditions.
Molybdenum disulfide:
Molybdenum disulfide is part of a group of materials known as “transition metal dichalcogenides” (TMDCs). Materials from this class are characterized by their chemical formula as MX2 in which M represents an atom of transition metal (groups 4-12 of the periodic table) and X is a chemical compound called a chalcogen (group 16). The chemical formula for molybdenum disulfide MoS2. MoS2 is relatively inactive. It is not affected by acids as well as oxygen. In appearance and feel, molybdenum disulfide resembles graphite. It is used extensively as a dry lubricant due to its very low friction and strength.
It is used for the two-stroke engines (such as motorcycle engines), bicycle coaster brakes, automotive CV, and universal joints and bullets.
MoS2 has an extremely low coefficient of friction. MoS2 has particle sizes within the range of 1 to 100 um. When we do a sliding friction test of MoS2 with the help of Pin on Disc tester with lower loads (0.1-2 N), it provides friction coefficients of <0.1.
MoS2 is often a component of blends and composites that require low friction and for instance, it can be added to graphite to increase adhesion. When combined with plastics, MoS2 can create a composite with increased strength. Self-lubricating composite coatings designed for high-temperature applications are made up of molybdenum disulfide and titanium Nitride by using chemical deposition of vapors.
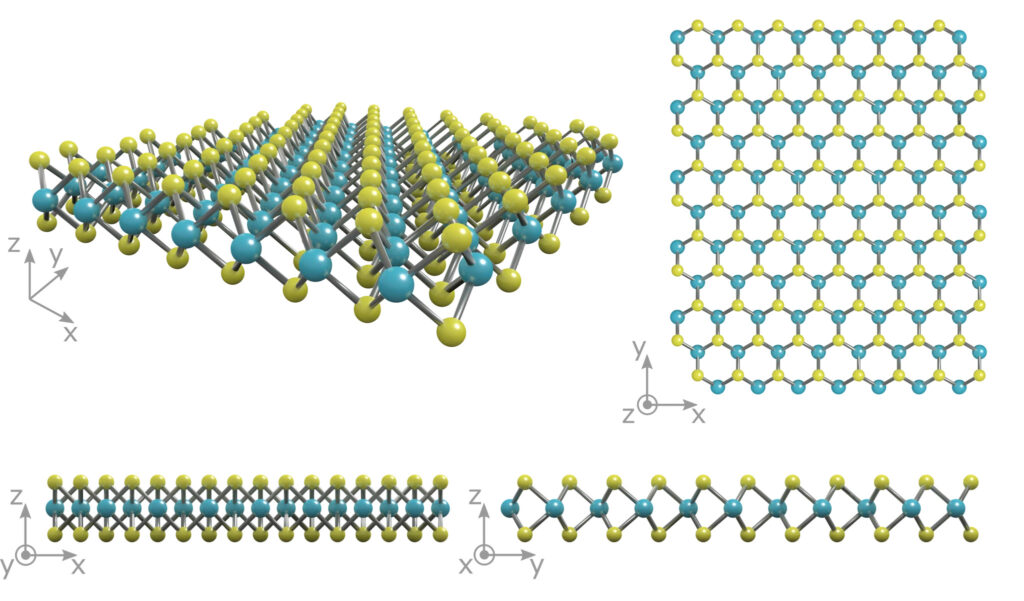
Figure: The crystal structure of monolayer MoS2 showing a layer of molybdenum atoms (blue) sandwiched between two layers of sulfur atoms (yellow).
The benefits of molybdenum disulfide’s solid oils include:
- Excellent adhesion
- A wide range of temperatures for service.
- Protection against fretting corrosion
- Reduced friction as loads increase
- Prevention of stick-slips
- Large capacity for load-carrying
Molybdenum disulfide’s solid lubricants can’t be used in humid conditions because moisture increases friction.
Hexagonal Boron Nitride:
In metal-forming processes, friction can lead to heat generation and adhesion, the pic up or accumulation of work materials, tools wear, inhomogeneous deformations, and poor quality surface. This is why suitable lubricants are essential, particularly when it comes to aluminum and aluminum alloys. However, due to the complexity increase of the parts formed with higher requirements for strength and the development of new alloys, lubricants are challenged with ever-increasing demands regarding temperatures stability, friction reduction, galling prevention, and surface protection.
Graphite is a solid lubricant that can be successfully employed in the hot and cold forming of aluminum, but it leaves dark staining on the surface of the part that is formed, and this means further grinding or polishing is needed.
One possible alternative to graphite could be hexagonal Hexagonal Boron Nitride (h-BN), also referred to as white graphite.
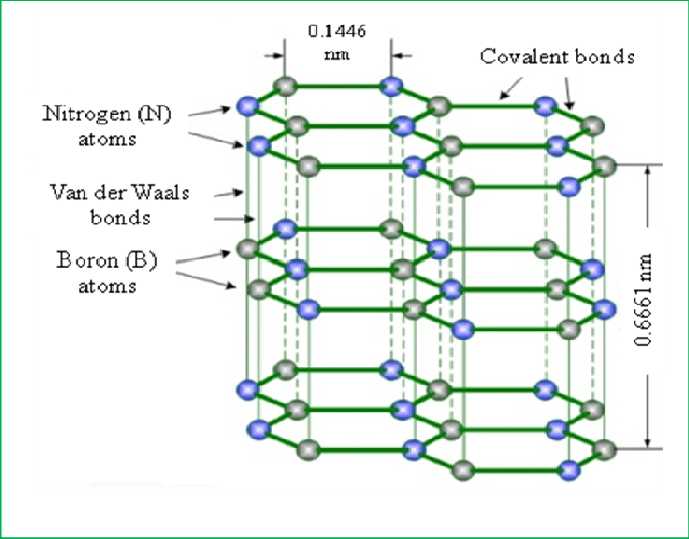
Figure: The structure of Hexagonal Boron Nitride (h-BN)
H-BN, as a lubricant, can be used to effectively replace graphite during Al-forming processes while simultaneously ensuring the purity of the surface and avoiding staining. Numerous studies have shown that the tribological properties that include wear and friction, lubrication-film stability, and the surface’s quality heavily depend on the size of the powder and the amount of Hexagonal Boron Nitride (h-BN). It is noticed that the highest performance was observed with 30um h-BN powder when added to grease in the range of 10 to 20 percent.
Tungsten disulphide:
Tungsten Disulfide (WS2) is a dry, solid powder that lubricates and is the most lubricious substance available. WS2 has a superior dry-lubricity (COF: 0.03) unmatched by any other substance such as Graphite and Molybdenum Disulfide (MoS2).
Tungsten Disulfide (WS2) is also utilized during make high-temperature and high-pressure applications. It has a temperature resistance range of up to -450 deg F (-270o C) to 1200 degrees F (650o C) in a standard atmosphere and a range of -305 deg F (-188o C) to 2400o F (1316o C) in a vacuum. The capacity to bear the load of the film is very high, reaching 300,000 psi.
As the powder of Tungsten Disulfide (WS2) is an extremely low coefficient of Friction (Dynamic at 0.03 and static @ 0.07), Applications are limitless and can be tested with any concept.
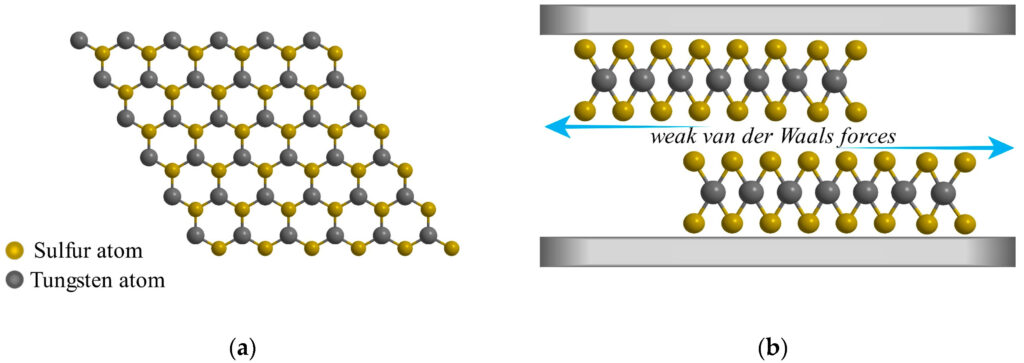
Figure: (a) Hexagonal structure of WS2 (top view); (b) The relative sliding between two single layers of WS2 (slide view).
Two well-established ways in which to use the WS2 powder is used are:
1) Mixing the powder of WS2 with semi-solid lubricants (such as grease, oil, and various chemical lubricants):
The powder is mixed from 1% to 15% (as necessary) with oil or grease. This increases the overall lubricity of the lubricant and will also enhance the High Temperature and Extreme Pressure properties of the semi-solid lubricants.
2) The WS2 powder is coated on the substrate that requires (dry) lubricity:
The powder is applied with spray (at 120 PSI) on the surface with dry (and cold) compressed air. It does not require binding agents, and the spraying process can be carried out at ambient temperature. The coated film is 0.5 microns in thickness. Suppose you want to apply it using a different method. The powder can be mixed with alcohol Isopropyl, and the resulting paste can be polished onto the substrate. The coating application is already widespread in different sectors like Automotive components, Racing Car Engine and other components, Aerospace parts, Bearings (Linear Ball, Roller Bal, etc.), Shafts, Marine parts, Cutting Tools, Blades, Slitters, Knives, Mold release, Precision Gears, Valve components, Pistons, Chains, Machinery components and many more categories.
Polytetrafluoroethylene (PTFE):
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene with numerous applications. The most commonly used brand name for PTFE-based materials is Teflon.
Polytetrafluoroethylene is a fluorocarbon solid (at room temperature), as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is not reactive, partly due to the strength of carbon-fluorine bonding that makes it so that it is commonly used in pipes and containers to protect against corrosive and reactive chemical substances. When used as a lubricant, PTFE reduces machines’ friction wear and energy consumption. It is often used as a graft for surgical procedures. It is also commonly used as a coating for catheters which hinders the capability of infective agents to adhere to the catheters and trigger infections in hospitals.
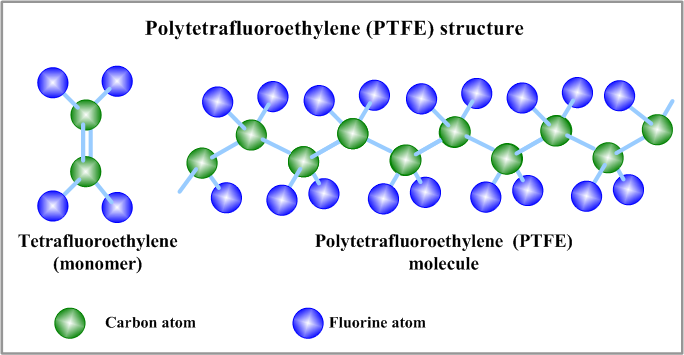
Figure: The structure of Polytetrafluoroethylene (PTFE)
The benefits of PTFE solids are:
- Good reduction in sliding friction.
- Good chemical resistance.
- Low load carrying capacity.
- The coefficient of friction is low at lower loads.
Applications:
Solid lubricants are beneficial in situations where conventional lubricants are inadequate, for instance:
Reciprocating motion:
Typical use is a sliding or a reciprocating motion that requires lubrication to reduce wear; for example in a chain and gear grease. Liquid lubricants can be squeezed out, whereas solid lubricants cannot let go, preventing corrosion, fretting and galling.
Ceramics:
Another possible application is that chemically active lubricant additives aren’t found on certain surfaces, including polymers and ceramics.
High temperature:
Graphite and MoS2 are lubricants that work at high temperatures and in oxidizing environments where liquid lubricants usually do not last. The typical use of these materials is fasteners that can be easy to tighten and remove after a prolonged period of high temperatures.
High contact pressures:
The lamellar shape is towards the sliding surface and results in a large bearing-load coupled with a low shear force. Most metal-forming applications which require the deformation of plastic use liquid fluids.
Methods for application:
Spraying/dipping/brushing:
Dispersions of solid lubricant as an additive in water, oil, or grease are frequently employed. Dry lubricant film may be sprayed when parts aren’t accessible for lubrication. Once the solvent has evaporated, the coating is cured and cooled to room temperatures, forming solid lubrication or friction reducer. Pastes are grease-like lubricants containing an extensive proportion of solid lubricants used to assemble and lubricate high-speed, slow-moving components.
Free powders:
Dry-powder tumble is a reliable technique for application. The bonding may be enhanced by prior phosphating the substrate. The use of free powders comes with limitations as their adhesion on the substrate is not enough to give any longevity for continuous applications. A brief time frame of improved slide conditions could be sufficient to enhance conditions for running-in of metal-forming processes.
Anti-friction coatings:
Anti-friction (AF) coatings are “lubricating paints” composed of fine particles of lubricating colors like moly disulphide, graphite, or PTFE mixed with binder. After the application and proper curing, these “slippery” (or dry) lubricants adhere to the metal’s surface, resulting in the dark gray solid film. Many dry film lubricants have particular rust inhibitors that offer outstanding corrosion protection.
Anti-Friction coating is applied for the below application areas and reasons:
- When galling and fretting are an issue (such as the splines, universal joints, and keyed bearings).
- When operating pressures exceed the capacity of ordinary greases and oils.
- Where smooth operation is desired like piston or camshaft, cases where clean operations are desired (AF coatings won’t collect dirt and other debris as oils and greases)
- Also where components are stored for long durations.
Self-lubricating Composites:
Solid lubricants like graphite, PTFE, MoS2, and other anti-wear and anti-friction additives are commonly compounded in polymers and other sintered materials. MoS2, for instance, is found in materials used for sleeves bearings and elastomers, O-rings, carbon brushes, etc.
Solid lubricants are incorporated into plastics to create the “self-lubricating” and an “internally lubricated” thermoplastic rubber. For instance, PTFE particles compounded in the plastic make a PTFE film on the mating surface, which results in a decrease in wear and friction. MoS2 is a compounded nylon that reduces friction, wear, and stick-slip. Additionally, it functions as a nucleating agent, resulting in the formation of a delicate crystal structure. The most common use for graphite-lubricated thermoplastics is in applications that operate in aqueous environments.
Leave a Reply
You must be logged in to post a comment.



Excellent research on lubricants
Excellent research on lubricants.