Administration of the project
Controlling the flammability of fuel
By Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2024
A minimum applied voltage of 6V is required to produce a volatile, flammable species from a nonvolatile ionic liquid.
• A potential fuel whose flammability can be controlled only when combustion is needed has been developed.
• Application of a voltage across an ionic liquid that contains 1-butyl-3-methyl imidazolinium as the cation and perchlorate as the anion converts a nonvolatile material into one that is flammable and generates a self-sustaining flame.
• Once the applied voltage is removed, formation of a flammable species ceases, and the ionic liquid reverts back to being nonvolatile.
High energy density fuels have been instrumental in ensuring that our transportation options such as airplanes and automobiles continue to provide the ability for individuals, industrial products and consumer goods to move from one location to another conveniently. These fuels are hydrocarbon based and exhibit flammability concerns that can become hazardous. One other issue is that current fuels are mainly derived from petroleum sources and are not in general, sustainable.
Researchers have been working to develop alternatives that are derived from renewable resources. In a previous TLT article,1 researchers worked to develop a more sustainable jet fuel based on lignin, a series of complex polymers that act as supporting materials in most plants. The currently used jet fuel, known in the U.S. as Jet-A, is a combination of various types of hydrocarbons and also has a sizeable aromatic content (approximately 8%-25% by volume). Lignin was an appealing raw material because of its cyclic nature, and the researchers produced a hydrocarbon fuel which displays a superior heat of combustion compared to Jet-A. Addition of 10% of the lignin-derived hydrocarbon to Jet-A produced a fuel that meets the specification limits for the incumbent jet fuel.
But the lignin-derived hydrocarbon is still flammable. Finding an approach for controlling flammability only when combustion is needed led researchers to examine room temperature ionic liquids. Michael Zachariah, distinguished professor of chemical engineering at the University of California Riverside in Riverside, Calif., says, “As combustion starts, there is no way at present to control the process by either accelerating it or slowing it down. In doing some work to evaluate ionic liquids as a propellant, we noticed that through electrolysis, the reactivity of the ionic liquid can be switched on and then off.”
Room temperature ionic liquids display high energy densities, which means they are suitable for use as fuels. These substances can be prepared with anionic components such as azides that are known to decompose to produce reactive flammable species. Another advantage of room temperature ionic liquids is they are relatively nonflammable and, in some cases, can be used as flame retardants. This makes them easier to handle than hydrocarbon-derived fuels.
Zachariah and his colleagues determined that a specific room temperature ionic liquid may have the potential to be used as a fuel where the flammability is controlled. He says, “Our objective was to find a thermally stable room temperature ionic liquid that can store an involatile energetic liquid as a nonflammable fuel, become flammable by increasing its volatility and extinguish its flame through decreasing its volatility.”
A class of room temperature ionic liquids has now been identified that meet these criteria.
Imidazolinium-based ionic liquids
The researchers evaluated a room temperature ionic liquid that contains 1-butyl-3-methyl imidazolinium as the cation and perchlorate as the anion. Zachariah says, “We knew that the aromaticity in the imidazolinium ring ensures that this substance is thermally stable and oxidation resistant. Previous studies also had shown that the imidazolinium ion will form an imidazole derivative in the vapor phase upon thermal decomposition but is not flammable when exposed to a hot-wire ignition or a butane flame. The important consideration is that the aromatic imidazole ring remains intact at elevated temperatures.”
But the researchers noticed that application of an applied voltage across 1-butyl-3-methyl imidazolinium perchlorate produces an electrochemical reaction that results in the formation of a volatile imidazolinium derivative that also is flammable and generates a self-sustaining flame. Upon removal of the applied voltage, formation of the volatile species stops, and as a consequence, the flame is extinguished.
Figure 4 shows how the concept of using an applied voltage will control the rate of flammability leading to a safer fuel. The imidazolinium-based room temperature ionic liquid is not flammable in the absence of an applied voltage as shown in the image on the left. Introduction of a voltage produces flammable species (top center image) which then produces a steady flame (upper right image). Removal of the applied voltage leads to a reduction in the flame (lower right image), and then full extinction of the flame (bottom center image).
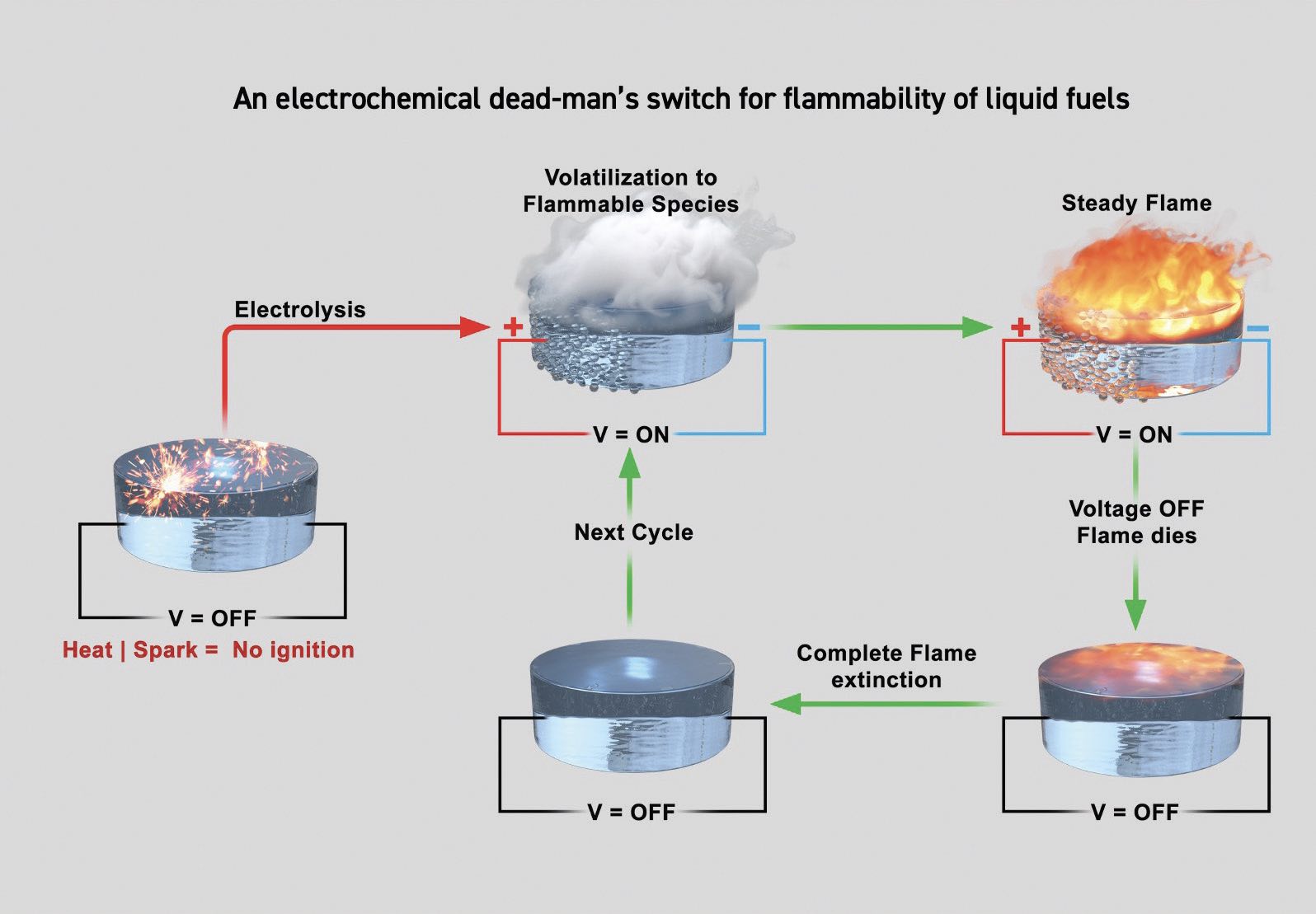
Figure 4. Applying a voltage to an ionic liquid based on an imidazolinium ion leads to the generation of a volatile species that is flammable and can support combustion. As the cycle proceeds and the voltage is removed, the flame is extinguished because the volatile species is no longer produced. Figure courtesy of the University of California Riverside.
Zachariah says, “We found that a minimum applied voltage of 6V was needed to produce the volatile, flammable species. The process was found to occur up to a voltage of 40V where the researchers determined from high-speed optical and infrared imaging that the reactive species form just at the cathode.”
Time-of-flight mass spectrometry was used to identify the volatile species formed. Direct sampling revealed that two radicals are generated during a reduction process at the cathode reaction that are volatile and flammable. Oxidation of the perchlorate anion occurs at the anode, but the resulting chlorate and chlorite anions are not flammable.
Zachariah says, “Our analysis indicates that the aromaticity of the imidazole ring is broken during the reduction reaction resulting in the formation of components responsible for undergoing combustion.”
The researchers will be evaluating other anions to determine if a similar process can occur at the anode. Zachariah says, “We also are working on an ionic liquid gel that can display the on/off capability to produce a solid that can be used as a fuel in a controlled manner.”
Additional information can be found in a recent article2 or by contacting Zachariah at [email protected].
REFERENCES
1. Canter, N. (2022), “Sustainable jet fuel based on lignin,” TLT, 78 (8), pp. 14-15. Available here.
2. Biswas, P., Wang, Y., Hagen, E. and Zachariah, M. (2023), “Electrochemical modulation of the flammability of ionic liquid fuels,” Journal of the American Chemical Society, 145 (30), pp. 16318-16323.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at [email protected].

Be the first to comment