Administration of the project
Improving the efficiency of catalytic converters
By Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2023
Placement of platinum atoms in a metal oxide is important in maximizing catalytic activity.
HIGHLIGHTS
• Positioning of single platinum atoms in certain locations on a cerium oxide support can influence one of the reactions conducted by a catalytic converter, oxidation of carbon monoxide.
• Embedding platinum atoms in the cerium oxide lattice also can improve catalyst performance in oxidizing carbon monoxide.
• This research shows the potential for placing platinum atoms in specific environments to facilitate catalytic activity and reduce the content of this precious metal required in a catalytic converter by at least 60%.
The focus on reducing automotive emissions is leading to the transition to battery electric and fuel cell-powered vehicles. While this trend is ongoing, internal combustion engine-powered vehicles will still represent the majority of automobiles operating globally into the future.
Catalytic converters are used to remove automotive emissions such as carbon monoxide from the exhaust emanating from the internal combustion engine. The catalysts used are precious metals based on palladium, platinum and rhodium. All three metals, while very effective, are very expensive, which has led to an increase in the theft of catalytic converters.
In a previous TLT article,1 researchers conducted experiments with palladium/platinum nanocrystals that modeled oxidation in a diesel engine to better understand how these two precious metals acted as catalysts. The model reaction used was the oxidation of the hydrocarbon, propene. Researchers found that the size of the precious metals has a significant effect on catalytic activity. Large nanoparticles between 10 and 20 nanometers displayed superior catalysis compared to smaller ones due to their ability to change shape, which leads to the formation of more active catalyst sites.
Fudong Liu, assistant professor in the department of civil, environmental and construction engineering at the University of Central Florida in Orlando, Fla., says, “Improving the efficiency of catalytic converters is an important goal in extending the operating life of catalytic converters and reducing operating costs. A goal that we have is to evaluate the efficiency of single atom catalysts supported on rare earth metal oxides. In particular, cerium is a good candidate because this rare earth metal absorbs and releases oxygen under the running conditions in an internal combustion engine. Many past studies have evaluated the catalytic activity of platinum on cerium oxide.”
Talat Rahman, distinguished Pegasus professor in the department of physics at the University of Central Florida, adds, “The factor that is most responsible for catalysis is undercoordination. Sites that can give electrons or get electrons are undercoordinated. This feature creates local environments where electrostatic forces are available to facilitate processes such as molecular tilting that can lead to the formation of oxygen-hydrogen bonds.”
Liu, Rahman and their colleagues figured that adjusting the local coordinating environments for single platinum atoms on cerium oxide supports may provide evidence for how a specific catalyst can perform in a particular reaction and provide information on how the catalyst structure affects performance.
Two new studies2,3 have now been published detailing how the reactivity of single platinum atomic catalysts changes based on their localized environment.
Carbon monoxide and ammonia oxidation
The initial study was focused on the oxidation of carbon monoxide. Liu says, “We found that evaluating the oxidation of carbon monoxide is the easiest reaction for determining the efficacy of the single atom catalyst because of the ease of characterizing the results.”
Catalyst preparation started with calcination of the cerium oxide at 800 C for 12 hours to ensure that the same support is used in all reactions. Calcination removes volatile substances present in the metal oxide.
Platinum atoms were then introduced onto the cerium oxide support at 550 C and 800 C. Liu says, “We chose 550 C because this is a standard temperature for preparation of this type of catalyst. Increasing the temperature to 800 C was conducted to accelerate aging of the catalyst and determine if its performance will differ.”
Through the use of several analytical techniques including hydrogen temperature programmed reduction, ultraviolet-visible light spectroscopy and high angle annular dark-field electron transmission microscopy, the structure of the single atom platinum/cerium oxide catalyst produced at the two temperatures was determined.
Liu says, “For the catalyst formed at 550 C, the single platinum atoms are located at cerium oxide edge sites and platinum-oxygen atoms display a coordination number of 5. This catalyst is very effective in oxidizing carbon monoxide but not in oxidizing ammonia. But at 800 C, the same catalyst is much more effective at oxidizing ammonia but is ineffective at oxidizing carbon monoxide. The catalyst prepared at the higher temperature has single platinum atoms situated at distorted cerium substitution sites on cerium oxide terraces. The coordination number of platinum atoms is 4.”
In the second study, further improvement in catalyst performance can be achieved by embedding platinum atoms in the cerium oxide lattice. Liu says, “We placed a single layer of platinum atoms in a position on the cerium oxide where they are not on the surface and not in the interior but somewhere in between in an embedded position. Catalytic activity in oxidizing carbon monoxide is 3.5 times higher than adsorbed platinum and 10-70 times higher than single platinum atoms.”
Aberration-corrected scanning transmission electron microscope images and structure models for platinum atoms are shown in Figure 1.
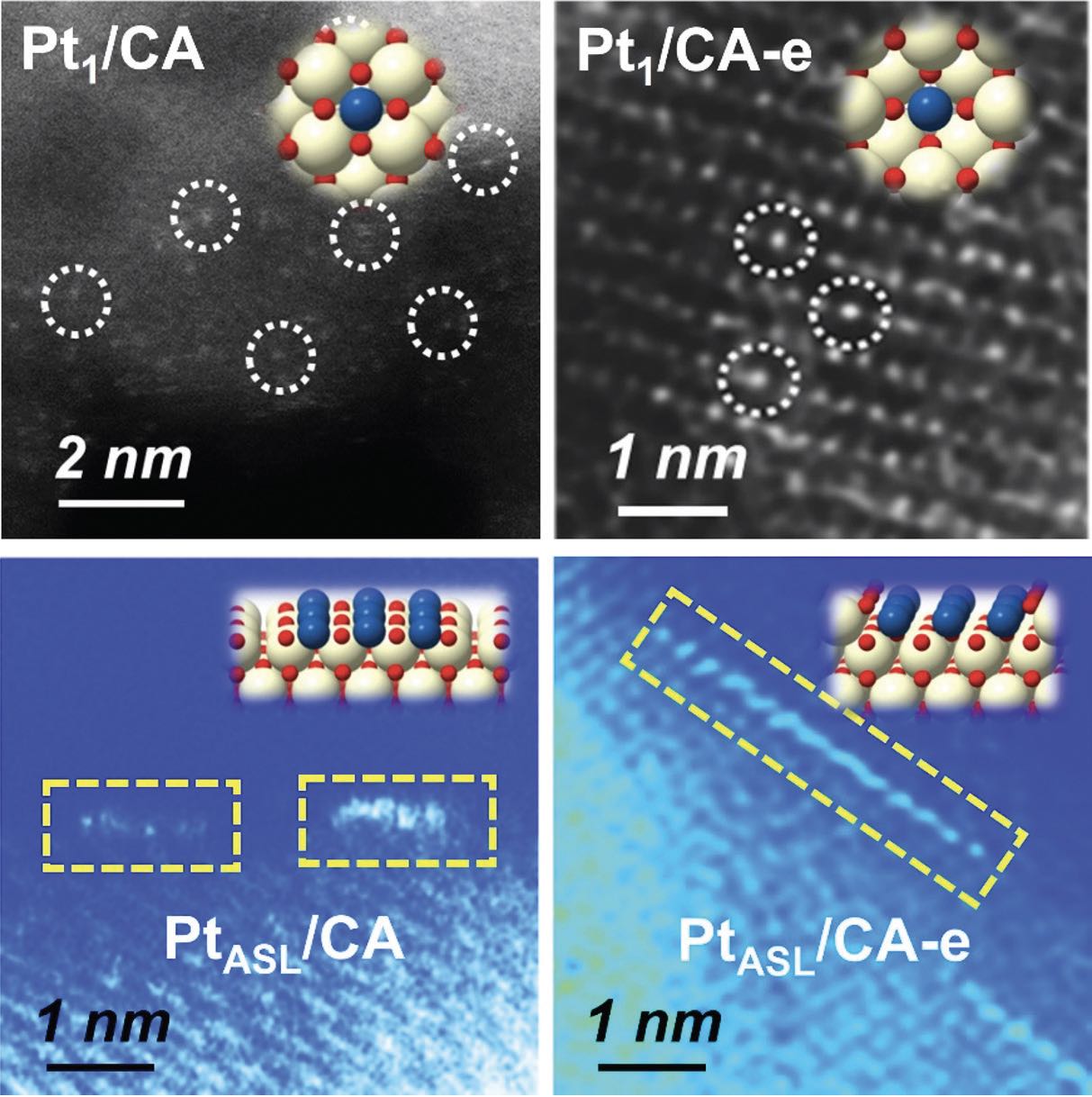
The researchers believe by preparing a specific environment for platinum atoms, they can reduce the quantity of precious metal needed to efficiently catalyze reactions that remove automotive emissions. Liu says, “We are now able to reduce precious metal content by 60% and obtain excellent catalytic activity. Our goal is to reduce content by up to 70%.”
Future work is underway to understand how the three-way catalyst used in the catalytic converter can be optimized not just for oxidations by platinum but for NOx reduction by palladium and rhodium. We also will be evaluating the ability of hexagonal boron nitride to reduce carbon monoxide to methanol.”
Additional information can be found in two recently published articles2,3 or by contacting Liu at [email protected] and Rahman at [email protected].
REFERENCES
1. Canter, N. (2020), “Determination of active vehicle exhaust catalyst sites,” TLT, 76 (11), pp. 14-15. Available here.
2. Xie, S., Liu, L., Lu, Y., Wang, C., Cao, S., Diao, W., Deng, J., Tan, W., Ma, L., Ehrlich, S., Li, Y., Zhang, Y., Ye, K., Xin, H., Stephanopoulos, M. and Liu, F. (2022), “Pt atomic single-layer catalyst embedded in defect-enriched ceria for efficient CO oxidation,” Journal of the American Chemical Society, 144 (46), pp. 21255-21266.
3. Tan, W., Xie, S., Le, D., Diao, W., Wang, M., Low, K., Austin, D., Hong, S., Gao, F., Dong, L., Ma, L,. Ehrlich, S., Rahman, T. and Liu, F. (2022), “Fine-tuned local coordination environment of Pt atoms on ceria controls catalytic reactivity,” Nature Communications, 13, Article Number: 7070, pp. 1-16.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at [email protected].



Be the first to comment