Founder of TriboNet, Editor, PhD (Tribology), Tribology Scientist at ASML, The Netherlands. Expertise in lubrication, friction, wear and contact mechanics with emphasis on modeling. Creator of Tribology Simulator.
Superlubricity between steel surfaces with glycerol/water mixture lubricant
It is estimated that the energy lost due to friction in industrialized countries equals to approximately 5% of their gross national products and it is clear, that reducing the friction is highly desirable.
The classical lubrication mechanism of the friction reduction has reached its fundamental limit (the friction of 0.01-0.04) and cannot reduce friction further. Therefore, other mechanisms are needed.
Superlubricity is a recently invented term in tribology, which is generally defined as the state, at which the friction coefficient is low. The definition of “low” is not quite clear, but it can be assumed less than 0.01. Due to the generality of the definition, the mechanisms of the superlubricity can be of various nature. At the nano scale, the structural superlubricity may occur, if the crystal lattices of the contacting bodies are incommensurate. Formation of nano bearings may also lead to the superlubricity state by changing the friction from sliding to rolling. The realization of the mentioned superlubric states typically requires very special conditions, such as vacuum, or materials, such as graphene, diamond-like carbon, etc. Therefore, translation of the superlubric state into macroscale and ordinary environment is a challenge.
Recently, researchers from Tsinghua University, Beijing reported a friction coefficient of as low as 0.006 at macro-scale between AISI-52100 steel parts using regular tribometer. As the lubricant, a glycerol-water mixture was used. Pure glycerol is a highly viscous, bio-compatible and environmentally friendly fluid with unique properties. Coupled with the discovered superlubricity properties and simplicity, it can be an attractive alternative to other typical lubricants, at least in certain applications.
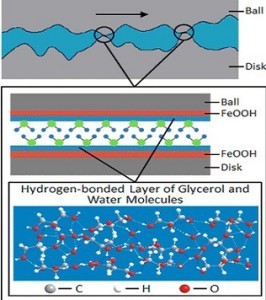
As it was found, on the metal surfaces, the glycerol and water molecules form a hydrogen-bonded layer, which is capable of carrying normal load. The layer is at the same time easy to shear in the direction of sliding rise to ultra-low friction. Moreover, it was found, that there is no chemical reaction taking place, unlike in the cases with typical additives used in industry. The absence of the chemical reaction reduces wear of the bare material. Glycerol intakes fluid from the surrounding and the viscosity of the glycerol-water mixture is highly dependend on the water concentration. Therefore, by controlling the environment humidity, it is possible to control the transiton from superlubrica to normal friction states, as discussed by the authors.
This lubrication method has a high potential in replacing the lubrication by means of classical hydrodyanmic theory.
The details can be found in the original article by Zhe Chen, Yuhong Liu, Shaohua Zhang, and Jianbin Luo, Controllable Superlubricity of Glycerol Solution via Environment Humidity.
Credit for image: Zhe Chen, Yuhong Liu, Shaohua Zhang, and Jianbin Luo, Controllable Superlubricity of Glycerol Solution via Environment Humidity.
Leave a Reply
You must be logged in to post a comment.

Thanks for sharing! This is a very interesting mechanism indeed. Did you also see their latest paper? Superlubricity of nanodiamonds glycerol colloidal solution between steel surfaces; http://www.sciencedirect.com/science/article/pii/S0927775715303228. Here they investigate the same base lubricant with added nanodiamonds to reduce wear.
What I am wondering is if this still works for a film thickness to RMS roughness ratio lambda << 1.
That’s a good question! We can try to test it in our lab! I have not seen yet the article you quoted, I will take a look now, thanks!
After reading the full article for an upcoming blog post it turns out this only works for mixed or hydrodynamic lubrication.
Interesting. What would be the reason for it to fail in boundary lubrication, is it due to wear of this hydrogen layer?
One of the hot topic in bearing industry these days is Hydrogen embrittlement. There is evidence that water can enhance crack propagation and fracture due to liberation of hydrogen. Also, there are many publications on the importance of using clean and dry oil. So I was wondering what will be the influence of water on corrosion, oxidation characteristics and on the service life.
I have a question rather than a comment: what ratio of glycerol to sea water would be ideal for a 30mm dia steel shaft rotating at 2500 rpm in a steel beating?