The article was created by Dr. Charchit Kumar, Postdoctoral Researcher and Teaching Associate, Laboratoire ICube (CNRS UMR 7357), Université de Strasbourg France.
Bio-Inspired Surface Replication
Replicating the complex surface topographies of nature
Approximately more than 400 million years of land plants evolution led to a vast diversity of structures on plant surfaces [1]. The surfaces of plant leaves are rarely smooth and often organized with a large variety of surface topographies, over a wide size range (from nano- to macro-scale), with distinct shapes, and including several hierarchical levels [1]. The species-specific and unique surface structuring of plant leaves, apart from surface chemistry, gives rise to various remarkable and inspiring functionalities, -which have drawn great attention, not only in biology but also in engineering disciplines, leading to get inspired, investigate and thus implement in engineering surface applications [2]. The plant cuticle, that constitutes the outermost layer of the plant body, provides various functional properties to plant leaves. For instance, anti-adhesive properties against insect’s attachments of the rubber tree (Hevea brasiliensis) leaves resulting from their fine cuticular folds (micro-structuring), and the insect trapping via slippery surface in some carnivorous plants that is caused by hierarchical structured architecture [3]. The well-known self-cleaning ability of the sacred lotus (Nelumbo nucifera) leaves arise from the remarkable de-wetting ability due to the complex hierarchical structure composed of papillate epidermal cells covered with hydrophobic nano-scale wax crystals [2]. The air retention ability of the floating fern Salvinia molesta is caused by the unique hairy surface morphologies (the Salvinia effect) [2]. Biological surface structures have been widely and well utilised as an inspiration to develop bio-mimetic and bio-inspired surfaces and devices.
In the last few decades, various techniques have been used to transfer the surface topographies of various plant leaves and, therefore, to emulate their properties induced by morphology onto technical materials such as polymers [4]. Compared to other replication techniques (sol–gel technique, atomic layer deposition, electroforming, and physical vapour deposition), replica moulding is relatively advantageous not only owing to its simple, straight-forward and inexpensive procedure but also because it allows direct employment of an original plant leaf as the master surface [4,5].
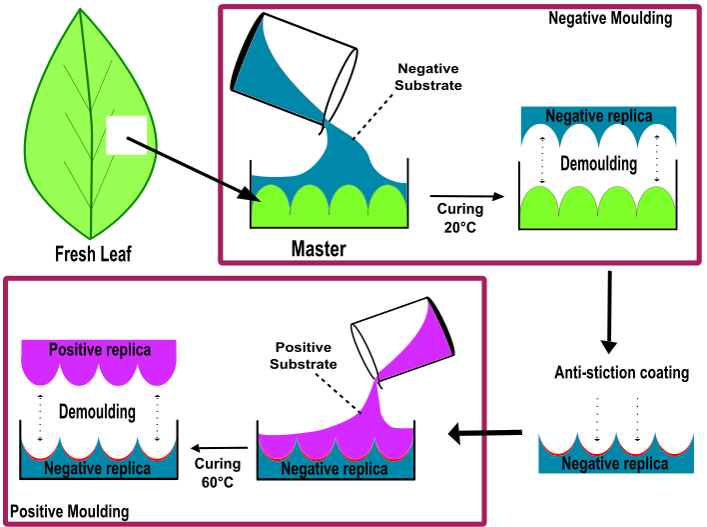
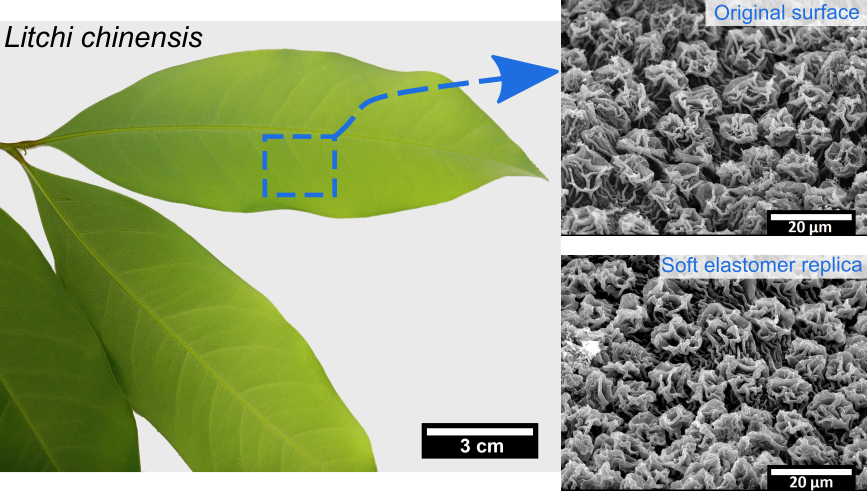
In general, the replica moulding technique follows a two-step double-casting process. At first, a negative replica is developed by pouring a liquid polymeric material onto a plant leaf (bio master template); afterwards, this negative replica is used to transfer the structures onto the second material (positive replica) [5]. The mutual affinity of both materials (negative and positive replica) may lead to the add-on of an intermediate anti-stiction film allowing for easy demoulding when producing the positive replica. Some researchers proposed a replication technique with using polyvinyl siloxane (PVS, common impression material employed in dentistry) as a negative replica and epoxy for the positive replica (hard material) [6]. Here, the quick curing (approximately 5 min) of PVS and its low adhesion to plant leaves is useful to prevent artefacts.
Another replication approach consists of utilising polydimethylsiloxane (PDMS) polymer to produce both negative and positive replicas; however, this approach involves an intermediate step of an anti-stiction treatment on the negative replica by organosilane monolayer deposition [7]. Usually, an anti-stiction surface treatment degrades with time and replication cycles (develop few accurate positive replicas). In a similar replication attempt, positive replicas were developed on a hard polymer PMMA (polymethyl methacrylate) from the PDMS negative replica [8]. Another replication technique comprises in the development of the negative replica on nickel template using sputtering and electroforming process, and then the structures from the negative replica were further transferred to acrylonitrile–butadiene–styrene copolymer and a UV-curable photopolymer [9]. A new replication strategy suggested using an epoxy-based polymer to produce negative replicas directly from original plant leaves, before transferring the surface structure onto final positive replicas made of PDMS [10]. In general, while utilising the plant surfaces for replication procedures, one has to keep in mind that plants leaves are delicate and highly sensitive to environmental conditions; temperature, pressure and replication material, therefore need careful treatment to achieve flawless replication. Finally, the accuracy and efficiency of a replication technique very much depends on the choice of materials for negative and positive replicas, respectively. It also assures the replication technique ability to generate fine and complex hierarchical topographies. However, the choice of final replica material solely depends on the application specific requirements. Furthermore, the precision level of replication accuracy needed for a particular application will largely depend on the type of application [5].
References
- [1] K. Koch, B. Bhushan, W. Barthlott, Diversity of structure, morphology and wetting of plant surfaces, Soft Matter. 4 (2008) 1943–1963. https://doi.org/10.1039/B804854A.
- [2] W. Barthlott, M. Mail, B. Bhushan, K. Koch, Plant surfaces: structures and functions for biomimetic innovations, Nano-Micro Letters. 9 (2017) 23. https://doi.org/10.1007/s40820-016-0125-1.
- [3] B. Prüm, R. Seidel, H.F. Bohn, T. Speck, Plant surfaces with cuticular folds are slippery for beetles, Journal of the Royal Society Interface. 9 (2012) 127–135. https://doi.org/10.1098/rsif.2011.0202.
- [4] D.P. Pulsifer, A. Lakhtakia, Background and survey of bioreplication techniques, Bioinspiration & Biomimetics. 6 (2011) 031001. https://doi.org/10.1088/1748-3182/6/3/031001.
- [5] C. Kumar, A. Palacios, V.A. Surapaneni, G. Bold, M. Thielen, E. Licht, T.E. Higham, T. Speck, V. Le Houérou, Replicating the complexity of natural surfaces: technique validation and applications for biomimetics, ecology and evolution, Philosophical Transactions of the Royal Society A. 377 (2018) 20180265. https://doi.org/10.1098/rsta.2018.0265.
- [6] K. Koch, A.J. Schulte, A. Fischer, S.N. Gorb, W. Barthlott, A fast, precise and low-cost replication technique for nano- and high-aspect-ratio structures of biological and artificial surfaces, Bioinspiration & Biomimetics. 3 (2008) 046002–046012. https://doi.org/10.1088/1748-3182/3/4/046002.
- [7] M. Sun, C. Luo, L. Xu, H. Ji, Q. Ouyang, D. Yu, Y. Chen, Artificial lotus leaf by nanocasting, Langmuir. 21 (2005) 8978–8981. https://doi.org/10.1021/la050316q.
- [8] R.A. Singh, H.J. Kim, J. Kim, S. Yang, H.E. Jeong, K.Y. Suh, E.-S. Yoon, A biomimetic approach for effective reduction in micro-scale friction by direct replication of topography of natural water-repellent surfaces, Journal of Mechanical Science and Technology. 21 (2007) 624–629. https://doi.org/10.1007/BF03026967.
- [9] S.-M. Lee, T.H. Kwon, Mass-producible replication of highly hydrophobic surfaces from plant leaves, Nanotechnology. 17 (2006) 3189–3196. https://doi.org/10.1088/0957-4484/17/13/019.
- [10] C. Kumar, V.L. Houérou, T. Speck, H.F. Bohn, Straightforward and precise approach to replicate complex hierarchical structures from plant surfaces onto soft matter polymer, Royal Society Open Science. 5 (2018) 172132. https://doi.org/10.1098/rsos.172132.

Be the first to comment