Administration of the project
Sequential nature of the electrochemical reduction of carbon dioxide
By Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2023
Chronoamperometric analysis was conducted to study how the electrochemical current produced during the reaction changes over time as the applied potential changes.
• The electrochemical reduction of carbon dioxide to a hydrocarbon such as ethylene is a useful approach for reducing emissions.
• The reaction pathway for converting carbon dioxide to ethylene is done through a sequential, three-step process.
• One of the reaction intermediates is either a carbon dioxide radical anion or carbon dioxide dimer radical anion.
Finding a means to utilize carbon dioxide in a beneficial manner has the potential to be an approach to reduce emissions and to improve sustainability. One logical strategy is to take carbon dioxide and, through a series of reactions, convert it to hydrocarbons such as ethylene, which are basic building blocks for producing raw materials used in lubricants.
A previous TLT article1 discusses the use of photoelectrochemical devices to facilitate the conversion of carbon dioxide to hydrocarbons in a process that emulates photosynthesis. Researchers determined the reason why a well-known photoelectrocatalyst, cuprous oxide, is not stable and exhibits a pronounced reduction in performance after only a few minutes when in use. The instability was attributed to cuprous oxide simultaneously undergoing oxidation to cupric oxide and reduction to elemental copper. This was determined to be a photocorrosion process that was minimized by introducing silver nanoparticles, which expedited electron transfer during the reduction of carbon dioxide.

Tobias Hanrath, Marjorie L. Hart ’50 professor in engineering in the Smith School of Chemical and Biochemical Engineering at Cornell University in Ithaca, N.Y., says, “Progress in gaining a better understanding of the electrochemical carbon dioxide reduction reaction over the past five years has been remarkable. Copper has emerged as the main catalyst because it can stabilize reaction intermediates and facilitate the formation of hydrocarbons. The challenge is expanding the reaction yield and improving selectivity. In experiments we conducted, at least five major products (hydrogen, carbon monoxide, formic acid, methane and ethylene) have been identified.”
Hanrath and his colleagues started to examine the mechanism of electrochemical reduction of carbon dioxide to better understand the complexity of the process. He says, “Our initial start came from a colleague who asked, what happens if the reaction is turned off and then turned on? Through experimentation, the answer was that the catalyst was refreshed after every cycle.”
This result led Hanrath to consider whether the element of time may play a role in better understanding the process. He says, “Most electrochemical reaction analysis is based on steady-state measurements taken during fixed time periods. We determined that studying the electrochemical carbon dioxide reduction over time may provide further details into the process and help to improve selectivity.”
Hanrath and his colleagues are now reporting on a study that relies on time-resolved measurements.
Electrochemical reaction at the electrode
Initial research involved using a two-compartment, glass H-cell that contained a copper foil working electrode, a silver/silver chloride reference electrode and a platinum mesh counter electrode. The one molar potassium chloride electrolyte was saturated with carbon dioxide prior to applying a voltage potential.
In applying increasingly negative potentials from -1.4 V to -1.8 V, the researchers initially identified carbon monoxide as the initial product in the electrochemical reduction process. A competing process, the hydrogen evolution reaction, takes place concurrently.
Increasing the negative potential then leads to the formation of ethylene and eventually methane. Carbon monoxide concentration drops because this species is an intermediate in the reduction process.
The researchers then used chronoamperometric analysis to study how the electrochemical current produced during the reaction changes over time as the applied potential changes. Hanrath says, “Initially, after the applied potential is changed, we observed that ions on the surface of the copper electrode rearrange in a similar manner to a capacitor and then the current generated evolves with time. There is an inverse relationship between current and time that is predicted by the Cottrell equation, devised over 120 years ago to better understand electrochemical reactions.”
Using Cottrell analysis, the researchers determined that the electrochemical reduction of carbon dioxide to ethylene proceeds through a sequential pathway. Hanrath says, “We believe the electrochemical reduction of carbon dioxide proceeds through a three-step process. After the initial electrochemical reaction on the electrode surface, the second step is a chemical reaction that takes place after the electrode. The third step is a second electrochemical reaction at the electrode that leads to the formation of ethylene.”
Figure 2 illustrates the process as carbon dioxide (represented by one black ball [carbon] and two red balls [oxygen]) is first converted to carbon monoxide (one black ball and one red ball) on the electrode surface followed by the formation of a dicarbon dioxide species, which is then converted to ethylene (two black balls and four grey balls [hydrogen]).
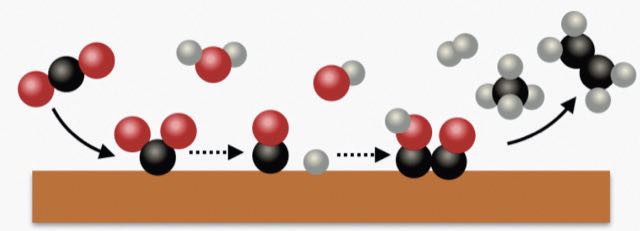
This analysis indicates that coupling of two carbons to form ethylene is not the rate determining step in the process. Hanrath says, “Our work supports previous studies that show ethylene formation occurs through either a carbon dioxide radical anion or carbon dioxide dimer radical anion intermediate.”
The researchers found that electrochemical reduction of carbon dioxide is kinetically favorable compared to the hydrogen evolution reaction. Hanrath says, “Capturing hydrogen with carbon monoxide and carbon dioxide increases the probability of ethylene formation.”
Future work will have the researchers looking to learn more about the fundamentals of the electrochemical reduction of carbon dioxide reaction. Hanrath says, “We need to determine if this process can be scaled up using commercially available electrolyzers such as a gas diffusion electrode and a membrane electrode assembly.”
Additional information can be found in a recent article2 or by contacting Hanrath at [email protected].
REFERENCES
1. Canter, N. (2022), “Conversion of carbon dioxide to ethylene and hydrogen,” TLT,” 78 (4), pp. 12-13. Available here.
2. DiDomenico, R., Levine, K., Reimanis, L. Abruna, H. and Hanrath, T. (2023), “Mechanistic insights into the formation of CO and C2 products in the electrochemical CO2 reduction—The role of sequential charge transfer and chemical reactions,” ACS Catalysis, 13 (7), pp. 4938-4948.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at [email protected].


Be the first to comment