Administration of the project
Conversion of carbon dioxide to ethylene
By Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2023
A new type of catalyst has been developed that produces a five-fold enhancement in selectivity for producing ethylene from carbon dioxide.
HIGHLIGHTS
• A hybrid catalyst, taking advantage of the complementary features of copper and nickel, has been developed to convert carbon dioxide to ethylene.
• Both the copper and nickel catalysts can operate at the same electrode and work under the same electrocatalytic condition.
• The most effective performance was found with a hybrid catalyst based on a copper to nickel ratio of 10:1.Ethylene is one of the basic building blocks used to manufacture numerous derivatives, including the synthetic lubricant base stock, polyalphaolefins (PAOs). The traditional process for manufacturing ethylene is by cracking naphtha and natural gas, which are both derived from petroleum.
Research is underway to find pathways for converting the greenhouse gas carbon dioxide into useful products that do not contribute to emissions. A previous TLT article1 describes work conducted to convert carbon dioxide first to carbon monoxide, which can then be used to produce hydrocarbons. The approach taken was to use electrolysis more efficiently through the use of a dynamic triple-phase boundaries electrolytic cell. A gas saturated electrolyte was moved through gas porous electrodes followed by efficient transfer to the catalyst surface, which contained silver nanoparticles deposited on a hydrophilic carbon fabric present on the cathode. This process generated a yield that is 10 times higher than previously reported.
The electrocatalytic carbon dioxide reduction reaction is emerging as another option for finding a way to convert this greenhouse gas into a useful derivative. Dr. Long Qi, associate scientist, chemical and biological sciences at the Ames National Laboratory in Ames, Iowa, says, “Production of ethylene through the use of cracking is a high temperature process that requires a large amount of energy and uses fossil fuel raw materials. Electrocatalytic carbon dioxide reduction is a much more sustainable process that uses a catalyst based on carbon, nitrogen, nickel and copper to produce ethylene at room temperature using electricity. It also is a process that can be scaled up to a commercial level easily.”
The catalyst that has traditionally been used in the electrocatalytic carbon dioxide reduction reaction is copper. The reason is that this metal has been found to facilitate the coupling of carbon-carbon single bonds leading to the formation of hydrocarbons and oxygenates with two and more carbon atoms. Efforts to improve the performance of copper have included tuning its crystalline plane, alloying with other elements, adjusting its oxidation state and surface ligand modifications.
Qi says, “Copper has two performance issues, which include the need for a high voltage overpotential. Copper also is not very selective in synthesizing a specific product. Using the electrocatalytic reduction process leads to the formation of multiple products, including hydrogen, and methane.”
To overcome the limitations of copper, a new type of catalyst has now been developed that produces a five-fold enhancement in selectivity for producing ethylene from carbon dioxide.
Hybrid catalyst
Qi, Wenyu Huang, faculty scientist at Ames National Laboratory and professor in the chemistry department at Iowa State University in Ames, Iowa, and their colleagues developed a hybrid catalyst based on copper and nickel that facilitates the electrocatalytic carbon dioxide reduction reaction to synthesize ethylene from carbon dioxide. Huang says, “Combining copper with nickel leads to the formation of a hybrid catalyst that takes advantage of the complementary catalytic properties of the two metals. Copper only slowly catalyzes the transformation of carbon dioxide to carbon monoxide but is very effective at converting carbon monoxide to ethylene. In contrast, nickel is very proficient at catalyzing the formation of carbon monoxide from carbon dioxide.”
The hybrid catalyst contains single-atom nickel anchored on nitrogen assembly carbon (Ni-NAC) and copper nanowires (CuNWs). Another important aspect is that both catalysts can operate at the same electrode and can work under the same electrocatalytic condition. The two catalysts were combined in a specific ratio, sonicated in hexane for two hours, purified, centrifuged and then dried in a nitrogen atmosphere. A catalyst paste was then prepared with polyvinylidene fluoride and n-methyl-2-pyrrolidone, and painted on a carbon paper, which was dried overnight under vacuum to produce a working electrode.
Figure 2 shows the structure of the two components in the hybrid catalyst and the process used to convert carbon dioxide to ethylene. The researchers evaluated several different ratios of copper to nickel to better understand the performance of the hybrid catalyst. Huang says, “We evaluated these hybrid catalysts because copper and nickel have different conversion rates. They need to match from a rate standpoint.”
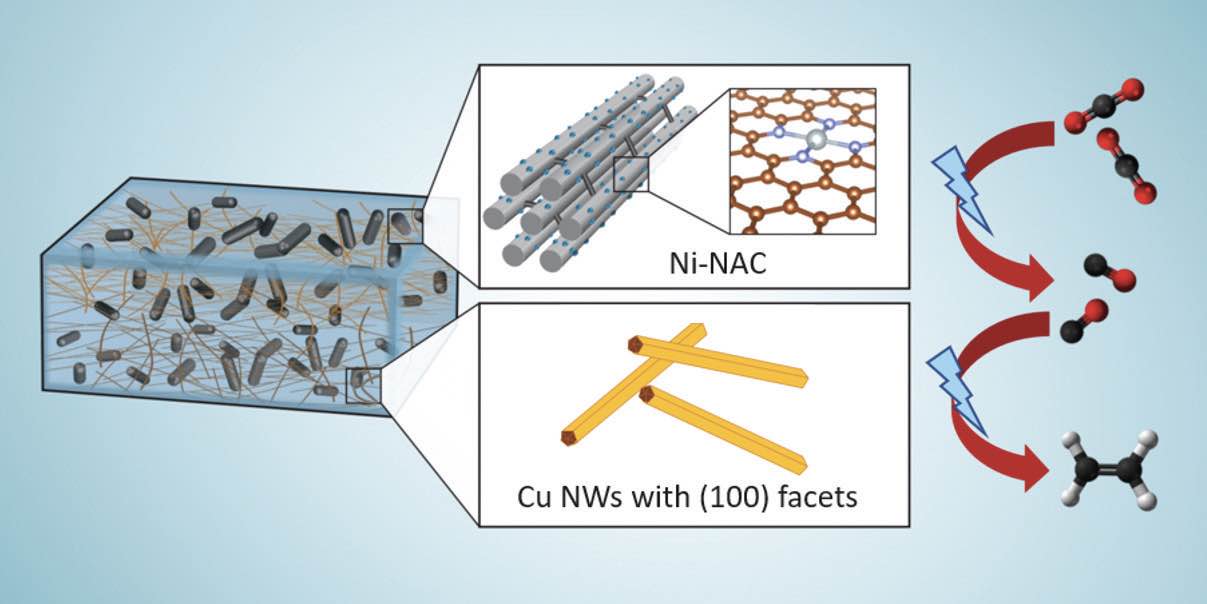
Figure 2. A hybrid catalyst based on nickel anchored on nitrogen assembly carbon (Ni-NAC—see top middle image) and carbon nanowires (Cu-NWs—see bottom middle image) was produced that can convert carbon dioxide to carbon monoxide and then to ethylene (right image). In the top middle and bottom middle images, the light blue atoms represent atomically dispersed nickel, the blue atoms represent nitrogen and the red atoms are carbon. In the right image, the black atoms are carbon, the red atoms are oxygen and the white atoms are hydrogen. Figure courtesy of the Ames National Laboratory.
Experiments conducted by the researchers showed that a 10:1 ratio of copper to nickel produced the most effective catalyst performance. The reason is the need for a high conversion of carbon dioxide to carbon monoxide to have sufficient intermediate available for use in producing ethylene in the second step of the process.
The researchers then adjusted the pH of the reaction from neutral to alkaline. Huang says, “The high pH conditions suppress side reactions such as the transformation of water to hydrogen.”
The hybrid catalyst used at the copper to nickel ratio of 10:1 under alkaline pH conditions produced a 66% Faradaic efficiency with over 100 milliamps per square centimeter at a -0.5 voltage potential.
The researchers will now work to improve catalyst performance closer to an efficiency of 70%. Long says, “We envision the development of a versatile platform where the surface area of the copper catalyst can be increased through the use of mesoscale pores. In addition, we are considering using this process to selectively produce oxygenates such as methanol and ethanol. The latter can be converted to ethylene through a dehydration process. A final objective is to evaluate a low grade of carbon dioxide in the flue gas, which is produced and discharged during combustion.”
Additional information can be found in a recent article2 or by contacting Laura Millsaps, communications manager at Ames National Laboratory, at [email protected].
REFERENCES
1. Canter, N. (2023), “Electrolysis of carbon dioxide,” TLT, 79 (2), pp. 16-17. Available here.
2. Yin, Z., Yu, J., Xie, Z., Yu, S., Zhang, L., Akauola, T., Chen, J., Huang, W., Qi, L. and Zhang, S. (2022), “Hybrid catalyst coupling single-atom Ni and nanoscale Cu for efficient CO2 electroreduction to ethylene,” Journal of the American Chemical Society, 144 (45), pp. 20931-20938.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at [email protected].


Be the first to comment